Artículos científicos
Effect of temperature on hatching and growth of cuban gar (Atractosteus tristoechus) larvae
Efecto de la temperatura en la eclosión y el crecimiento de las larvas de manjuarí (Atractosteus tristoechus)
1*Yamilé Comabella, 2Andrés Hurtado, 3Javier Canabal, 1Tsa¡ García-Galano
1 Centro de Investigaciones Marinas. Universidad de La Habana. Calle 16 114 e/lra y 3ra, Miramar, Playa, Cuba. * ycomabella@yahoo.es
2 Centro de Reproducción de la Ictiofauna Indígena, Ciénaga de Zapata, Cuba.
3 Knowles Animal Clinics, Florida, United States.
Artículo recibido: 20 de marzo de 2013
Aceptado: 22 de enero de 2014
ABSTRACT
Hatching success, growth, development and survival of Cuban gar (Atractosteus tristoechus) larvae were examined at different temperatures (26, 28 and 30 °C) up to 18 days after hatching (DAH). The time to hatching was inversely related to the incubation temperature (87, 100 and 111 h). Larval survival at the time of hatching was significantly lower at 30 °C (50.3 %), coinciding with the highest larval mortality (30.2 %). Growth rates were 1.75 mm d-1 - 10.4 % d-1 at 26 °C, 1.30 mm d-1 - 10.2 % d-1-1 at 28 °C and 1.40 mm d-1 -10 % d-1 at 30 °C. Three similar critical periods were identified: 0-6, 7-11 and 12-18 DAH. During the first period, a similar increase in weight and a significant increase in total length occurred, mainly at 30 °C, indicating a more efficient reconversion of the yolk reserves. Later, growth was equally slow, corresponding with the transitional period from endogenous to exogenous feeding, indicating a similar physiologic pattern regardless of incubation temperature. Weight and length increased during the last period, with the greatest increase at 26 °C in contrast with the lowest gain at 30 °C. The inflexion points of many morphometric characters and the developmental stages accelerated with the increasing temperature. Although it was impossible to determine the optimal temperature, it was evident that 26 °C favored hatching success and larval growth.
Key words: Fish larvae, incubation temperature, gar, hatching, allometry, development, growth.
RESUMEN
Se examinaron el éxito en la eclosión, crecimiento, desarrollo y supervivencia de larvas de manjuarí (Atractosteus tristoechus) a diferentes temperaturas (26, 28 y 30 °C) hasta los 18 d después de eclosionadas (DDE). El tiempo de eclosión fue inversamente proporcional a la temperatura de incubación (87, 100 y 111 h). La supervivencia larval en el momento de la eclosión fue significativamente menor a 30 °C (50.3 %), coincidiendo con la mayor mortalidad larval (30.2 %). Las tasas de crecimiento fueron 1.75 mm d-1 - 10.4 % d-1 a 26 °C, 1.30 mm d-1 - 10.2 % d-1 a 28 °C y 1.40 mm d-1 -10 % d-1 a 30 °C. Se identificaron tres períodos críticos similares: 0-6, 7-11 y 12-18 DDE. En el primero ocurrió un incremento similar en peso y un incremento significativo en la longitud total, principalmente a 30 °C, lo cual reveló una reconversión más eficiente de las reservas vitelinas. Posteriormente, el crecimiento fue igualmente lento, correspondiéndose al período de transición de la alimentación endógena a exógena, indicando un patrón fisiológico similar e independiente de la temperatura de incubación. En el último período, tanto el peso como la longitud aumentaron, con el mayor incremento a 26 °C, contrario a la menor ganancia a 30 °C. Los puntos de inflexión de muchos de los caracteres morfométricos y las etapas de desarrollo se aceleraron con el incremento de la temperatura. Aunque fue imposible determinar la temperatura óptima, se hizo evidente el beneficio para el éxito de eclosión y el crecimiento larval a 26°C.
Palabras clave: Larvas de peces, temperatura de incubación, lepisosteidos, eclosión, alometría, desarrollo, crecimiento.
INTRODUCTION
Water temperature has been shown to be one of the most important factors influencing growth, survival, feed efficiency and development offish larvae in both laboratory and natural environments (Seikai et al. 1986, Kling et al. 2007, Le et al. 2011). It affects the metabolism, activity, structure and quality of the developing embryo and larvae (Saka et al. 2005, Ahmad et al. 2011, Frisk et al. 2012). Fish generally present a temperature optimum for growth and survival that can change with age and size (Jonassen et al. 1999), and normally varies among species (Rana 1990a). Each species has an optimal range for the best developmental success depending on its ecology and life history.
Since temperature is generally the most influential and variable environmental parameter, and is also the most controllable in hatcheries, it has been the most thoroughly researched factor influencing fish development (Wang et al. 1987). Furthermore, its effects on growth have been described for several fish species including cod (Imsland et al. 2007, Hanna et al. 2008), halibut (Steinars-son and Björnsson 1999), brown flounder (Huang et al. 2008), mackerel (Mendiola et al. 2007b), pinfish (Reber and Bennett 2007), trahira (Petry et al. 2007), sea bass (Georgakopoulou et al. 2007, Dülger et al. 2012), yellowtail kingfish (Abbink et al. 2012) and others.
However, the relationship among incubation temperature, embryonic development and larval growth of Cuban gar has not yet been described. Cuban gar (Atractosteus tristoechus) is an endemic freshwater fish that inhabits the western region of Cuba, primarily Ciénaga de Zapata. The species was listed as vulnerable in 1999 (Pérez et al. 1999) because, apart from restrictions on its habitat, other factors such as habitat loss and ecological alteration have contributed to a decline in the natural populations. For these reasons, interest in its cultivation has increased in order to preserve and restore natural populations through stocking of cultured individuals. This study was undertaken to provide information on the effect of three temperatures (26, 28 and 30 °C) on hatching performance, growth and development of Cuban gar larvae.
MATERIALS AND METHODS
Experimental design, sampling and measurements
Cuban gar (Atractosteus tristoechus) eggs were obtained from the induced spawning of one female and three males kept in captivity at the Center for Native Ichthyofauna Reproduction located in the peninsular region of Zapata, Cuba. Breeding adults were placed in a 3 x 2.5 m concrete pond with water to a depth of 50 cm. In order to favor the spawning behavior of the lepisosteids (León et al. 1978, Simon and Wallus 1989), artificial branches were placed throughout the pond to provide spawning substrates. A first injection of luteinizing hormone-releasing hormone analog (LHRH-A; 25 /µg ml-1 - Argent Chemicals, USA) was given to the broodstock, and a second injection was given 16 h later. Courtship and spawning occurred 9 h after the second injection. Fifteen minutes after spawning, nine artificial branches with 100 adhesive eggs on each were selected. The branches were carefully removed and placed in nine 15 L circular fiberglass tanks, where the trials took place for up to 18 d after hatching (DAH). The experiment was carried out in triplicate at three different constant temperatures (26, 28 and 30 ±1°C). The eggs taken from the broodstock pond and transferred to the experimental tanks were adapted gradually (four hours) to each test temperature. Room temperature was controlled (24 ±1°C). Eggs and larvae were reared under an 08:00 to 20:00 h light regime, oxygen levels were maintained above 6 ppm and 50 % of the water was exchanged daily in each tank after cleaning the bottom. After hatching, the larvae were fed live Moina ad libitum three times a day (09:00, 14:00 and 19:00 h).
Hatching time H50 (50 % larval hatching) was determined through hourly observations of each experimental unit. Embryonic, pre-hatching and posthatching mortalities were recorded at H50. Larval survival was verified at that moment, as well as at the end of the experiment.
From hatching up to 18 DAH, two to three larvae per tank were selected randomly, anaesthetized with tricaine methanesulphonate (MS 222), individually weighed on an Ohaus scale (±0.1 mg) and preserved in a 70 % ethanol solution for later examination. Seventeen morphometric characters (total length (TL); standard length; snout length; head length (HL); predorsal and preanal length; trunk and tail length; pectoral and pelvic fin length; cephalic height; pectoral, preanal and postanal height; caudal peduncle height; snout and head width) were recorded for each specimen using an ocular micrometer and digital caliper (±0.1 mm), considering the criteria defined and illustrated by Simon and Wallus (1989) for lepisosteid larvae. The relationship between incubation temperature and developmental larval stage, proposed by Comabella et al. (2010), was also analyzed.
Data analysis
Growth rate (GR, mm d-1) was calculated as [TL2 - TL1]/(t2 - t1), where TL2 and TL1 are the total length at times t2 and t1, respectively. The Specic Growth Rate (SGR, % /d-1) was calculated lated as 100 [In W2-ln W1] /(t2 - t1), where W2 and W1 are the wet body weights at times t2 and t1, respectively.
Data for H50, final survival and growth rates were analyzed with a one-way analysis of variance (ANOVA) to detect differences (p < 0.05). Significant ANOVAs were followed by a post hoc multiple comparison test (Tukey test). Prior to the ANOVA analyses, data expressed as percentages were arcsine square-root-transformed. A regression analysis was used to describe larval growth. The analyses were carried out with the Statistica program for Windows (Stat-Soft, Tulsa, Okla.).
Allometric growth was calculated as a power function of X (X = TL (or HL for widths)) using non-transformed data: y = αXb, where y was the measured character, a the intercept and b the growth coefficient (Fuiman 1983). The equations were established from regressions carried out on log-transformed data, using TL or HL as the independent variable (Gisbert 1999; Gisbert et al. 2002). When growth is isometric, the growth coefficient is b = 1 for length, height or width and is b = 3 for weight when compared with X (Osse & Boogart 2004). Allometric growth was positive when b was > 1 or 3 and negative when < 1 or 3. Diphasic growth may be described with two different growth curves. The X value, where the slope changes, is called the inflexion point. Inflexion points were determined using iteration procedures according to Snik et al. (1997), Gisbert (1999) and Gisbert et al. (2002). The xy data set was sorted according to an increasing X. Regression lines were calculated for Xmin until X intermediate, and for Xintermediate until Xmax, where Xintermediate varied iteratively from Xmin +2 to Xmax -2. Also, t tests were applied to check whether the growth coefficients for Xmin Xintermediate and Xintermediate Xmax differed significantly. The Xintermediate value that resulted in the largest t was defined as the inflexion point. Growth coefficients were compared statistically with a t-test. The accepted significance level was p < 0.05.
RESULTS
Survival and hatching success at various incubation temperatures
The time required to reach H50, the mortalities and the larval survival at this moment and at various constant temperatures are presented in Table 1. The time to hatching (H50) was inversely related to the incubation temperature. Larval survival at the time of hatching was significantly lower at 30 °C (50.3 %), and coincided with the highest post-hatching larval mortality (30.2 %).
Incubation temperature effect on larval growth
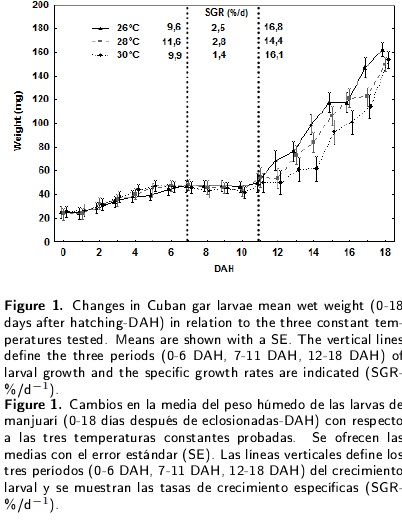
Although final larval survival was high (> 90 %) and no significant differences were found for the tested temperatures (Table 1), larval growth varied among the treatments during the first 18 d of Cuban gar life. Figure 1 shows the weight growth of the A. tristoechus larvae reared at the tested temperatures, with a 6 - 6.5 fold increase in weight from 0 to 18 DAH. The increase in total weight was described by the equations: TW(26°C)= 22,593 x exp(0,1006 x DAH), TW(28°C)= 23,867 x exp(0,0913 x DAH) and TW(30°C)= 25,736 x exp(0,0783 x DAH). The specific growth rate averaged 10.4 % d-1 (26°C), 10.2 % d-1 (28°C) and 10 % d-1 (30°C) during the experiment. It is possible to distinguish three similar periods of larval growth for each temperature: 0-6 DAH, 7-11 DAH and 12-18 DAH. This rate increased during the first and third periods. However, during the 7-11 DAH period, the increase in body weight was slow in all the treatments. The greatest specific growth rates were recorded for 28°C during the first and second periods and for 26 °C during the third.
Regarding the total length growth (Figure 2), the increase was 3 and 3.3 fold for 28 and 30 °C respectively, and 8.4 fold for 26 °C due to a smaller larval size at the time of hatching at this temperature. The equations for the relationship between the days after hatching and the total length were: TL(26°C)= 10,337 + 1,3862 x DHA TL(28°C) = 12,995 + 1,1673 x DAH and TL(30°C)= 14,338 + 1,0165 x DAH. During the early larval development, the growth rate averaged 1.75 mm d-1 (26°C), 1.30 mm d-1 (28°C) and 1.40 mm d-1 (30°C). A three-period pattern similar to that mentioned above was observed, with the greatest growth rates during the first and second periods at 26°C, as well as during the third at 30°C. Also evident was the slow increase in body length during the 711 DAH period in all the treatments. A significant interaction was recorded between temperature and larval age for the total length and the wet weight.
The relationships between daily growth rate and specific growth rate vs temperature are presented in Figures 3 and 4 respectively. The temperature had a significant effect on both growth rates during the larval period, with the highest values recorded for the 26°C treatment. However, the optimal temperature for Cuban gar larvae growth was impossible to determine.
Wet weight growth was negatively allometric, biphasic and had the same inflexion point (14 DAH) in the three temperatures (Table 2). Of the 17 morphometry characters measured, only the standard length presented isometric growth as a function of total length during the early stages of development in all the tested temperatures. In addition, isometric growth was observed in another five characters (predorsal and preanal lengths, preanal and postanal heights, and tail length) at 28°C and 30°C. In contrast, the other body proportions and growth coefficients changed considerably during this period in all the treatments, showing biphasic growth patterns.
The head and snout lengths had a positive allometric growth during larval development with inflexion points at 6 DAH (26°C and 28°C) and 3 DAH (30°C). The growth in head height and in head and snout widths was negatively allometric, with a remarkable anticipation of the inflexion point for head height at 30°C. The trunk length growth was negatively allometric as well, with inflexion points that advanced from 3 DAH at 26°C to 1 DAH at 30°C. A similar pattern was found for the pectoral height, but the anticipation of the inflexion point was from 5 DAH at 26°C to 3 DAH at 30°C. The preanal-postanal heights and predorsal-preanal lengths showed a negative allometry only in the larvae reared at 26°C. The pectoral and pelvic fins increased in length from hatching to 18 DAH with a positive allometric growth (except for the pectoral fins at 26°C), and the same anticipation of the infexion points was evident with the increase in incubation temperature. Finally, the tail length growth in the early larvae showed the same isometric trend as the other length characters presented in Table 2, except for 26°C where a positive allometric growth appeared with an early infexion point. The peduncle height showed a negative allometric growth throughout the 18 days of the experiment in all the treatments.
Incubation temperature effect on larval development
Incubation temperatures also influence the morphological development of larvae. According to Comabella et al. (2010), three stages of Cuban gar larval development were established: Stage 1- Attached, Stage 2- Transitional and Stage 3- Free Swimming. Figure 5 presents the relationship between larval development stage and age at different temperatures. Larval development accelerated at 30°C as, from 3 DAH onwards, the larvae were in the second stage, whereas this occurred one day later at the other two temperatures. This difference was even more marked in the transition to the third stage which was reached on 7 DAH at this temperature and on 10 DAH (28°C) and 12 DAH (26°C) in the other treatments.
DISCUSSION
Broodstock spawning, embryo development, survival and growth of fisauthors considered that these physiologicalh occur within a narrow range of water temperatures that may differ among species, ages and body sizes, and may reflect their temporal and spatial distribution in the field (Imsland et al. 1996, Saka et al. 2005, Teletchea et al. 2009). Incubation temperature has a direct effect on the timing of embryonic development and determines hatching efficiency, as was observed in Cuban gar larvae. The first differences were observed in the time of hatching (H50), which was inversely related to the incubation temperatures, as has been reported for other species (Rana 1990a, Kamler et al. 1994). In comparison with other lepisosteids, Márquez (1998) found that 75-80 % of Atractosteus tropicus larval hatching occurred between 21 and 80 hours post fertilization at 25, 30 and 35 oC. In the case of A. spatula, this process took place from 50 to 57.5 hours post fertilization at 27-28°C (Morales 1999, Mendoza et al. 2002). In the present study, the time required to reach H50 varied from 87 to 111 h post fertilization at our temperatures (26, 28 and 30°C), indicating a larger embryologic period for A. tristoechus. During this critical moment in the life cycle of the fish, signifcant diferences were observed in post-hatching larval mortality. The greatest percentage at 30°C coinciding with the lowest larval survival shows the harmful effect of this temperature on hatching success. However, the embryonic and pre-hatching mortalities were not statistically different at the tested temperatures, and no deformities in larvae at the time of hatching were observed for the highest temperature. Sometimes, when incubation temperatures are outside the optimal range, development appears to be inhibited at the early stages, generally near the time of gastrulation (Wang et al. 1987). This results either in embryo death or in the development of abnormalities that may increase mortality (Hart Purser 1995). This pattern has been observed in white sturgeon as well as in other species (Wang et al. 1987). Our data indicate that a temperature of 30°C increases mortality at the time of hatching, but it cannot be associated with a supra-optimal temperature that may cause drastic effects in embryo development, such as spinal and jaw deformities and a smaller fish size at the time of hatching, as has been described for other species (Georgakopoulou et al. 2010). Also, larval survival in all treatments of our experiment was high and similar to that reported for other Atractosteus species of 80-90 % (Márquez 1998, Morales 1999, Mendoza et al. 2002).
Temperature has been proven to affect almost every aspect of the early development of fish: hatching, initial feeding time (Huang et al. 2008), yolk conversion efficiency, and size and body condition at first feeding (Ojanguren et al. 1999). These authors considered that these physiological responses reveal the controlling effect of temperature on metabolic processes through thermal dependence on enzymatic activity. As poikilotherms, fish cannot maintain a body temperature different from the surrounding water; therefore, temperature affects their biochemical and physiological activities. Many studies have reported species-specific metabolic and behavior responses to thermal acclimation, indicating changes in hematological pattern (Das et al. 2009, Martins et al. 2011), excretion (Smatresk Cameron 1982b, Nerici et al. 2012b), mobility and swimming (Hanna et al. 2008, Martin et al. 2011), feeding (Bailey Alanärä 2006), bone ontogeny (Loffler et al. 2008, Georgakopoulou et al. 2010), muscle cellularity (Carey et al. 2008), rate of oxygen consumption (Barnes et al. 2011, Nerici et al. 2012a), and even the air-breathing frequency in facultative air breathers such as lepisosteid fish (Rahn et al. 1971, Smatresk Cameron 1982a,b).
In the present study, three similar critical periods for larval weight and length growth were identified for the three tested temperatures. The first period (0-6 DAH) was characterized by a similar increase in weight at all temperatures and a significant increase in the total length mean, with the highest growth rates at this time in relation to all the days of the experiment. Specifically in the case of total length, larvae at the time of hatching seemed to be significantly smaller at lower temperatures than at higher ones. The size of newly-hatched larvae of several fish species has been shown to be positively and negatively influenced by temperature (Mendiola et al. 2007b). At the time of hatching, larval size may decrease as incubation temperature increases (Kamler et al. 1994). Larvae could convert more of their yolk to body tissue at higher temperatures (Rana 1990b). Others could reach a maximum body size at intermediate temperatures (Sun et al. 2006, Mendiola et al. 2007b). In our case, this difference was found only at the time of hatching, as the total larval length in all the treatments was similar after 24 h.
During the first days, larvae are in a lecithotrophic phase, remain vertically adhered to vegetation and feed exclusively off the yolk sac (Comabella et al. 2010). At the beginning, larvae may increase in length due only to the use of the yolk sac elements, while weight remains almost stable as there is no uptake of external nutrients. This was confirmed in a previous study (Comabella et al. 2006) that observed a significant increase in larval protein concentration starting on 9 DAH, together with an input of new protein sources from exogenous feeding. Although no differences in weight were recorded in the three tested temperatures during this first period, the gain in total length was significantly higher with the increase in temperature. This could reveal a more efficient use and reconversion of the yolk reserves into an increase in body size at the highest temperature. Similar results have been reported for white sturgeon (Wang et al. 1987) and Atlantic salmon (Ojanguren et al. 1999). These findings indicate that larvae have the capacity to absorb endogenous nutrients differently under different water temperature conditions, and this means that the rate of absorption of the endogenous nutrients can be controlled by water temperature. However, further study is needed to determine which other environment parameters affect the larval absorption of reserves under rearing conditions.
After this period, from 7 to 11 DAH the body weight and total length increased slowly in the three treatments, corresponding to a lecithoexotrophic -exotrophic stage. The process of obtaining food begins at this moment, though the yolk reserves are still used. This is the transitional feeding period that is defined as an interval in which feeding ability develops and feeding starts, with some reserves still present to meet the energetic demands of prey capture (Moteki et al. 2001, Williams et al. 2004). This period is considered to be a critical and vulnerable time in the initial ontogeny of fish due to predation and competition for food (Balon 1985; Coughlin 1991, Makrakis et al. 2005). It has also been considered as the period of greatest change in larval appearance, and in organ and structure development (Williams et al. 2004), and when a decrease in growth could occur (Gisbert et al. 2002, Geerinckx et al. 2008). Our results indicate that this change from endogenous to exogenous feeding, which ends with the exhaustion of the yolk reserve, follows a similar physiologic pattern regardless of incubation temperature.
After this critical transition period, larvae are exotrophic and are able to detect food items and prey efficiently in the water column. This period is characterized by a significant increase in daily weight and total length due to an effective assimilation of external nutrients. However, this pattern was inverted in the case of our temperatures, as the greatest increase in weight and total length was obtained at 26 °C and the lowest at 30 °C. Since yolk represents the main source of energy and materials for sustaining growth in early embryos and pre-feeding larvae, the effect of temperature on growth and development processes is well seen in this phase. At this time, conflicting demands and constraints by other organic functions appear (e.g. locomotion, feeding, social interaction), and metabolic processes and physiological activities may be affected. This could suggest that long-term exposure of Cuban gar larvae to 30 °C may induce some type of stress. This thermic stress could impede the appropriate obtaining and/or assimilation of nutrients, resulting in a decreased growth. It is also possible that the composition, mobility or survival of live prey could be affected by this higher temperature. However, no significant changes in behavior, survival, health or oxygen demand were observed at this higher temperature, compared with the other treatments. In the present study, larval growth was examined only in terms of length and weight. Further detailed analyses should be carried out to monitor daily food and oxygen consumption, and other parameters that may explain the reason for this larval growth pattern once the yolk reserves are finished.
An uncommon statistically significant interaction between temperature and larval age in Cuban gar was also evident. Previous studies with japanesse flounder (Seikai et al. 1986), halibut (Jonassen et al. 1999), pollack (Ruyet et al. 2006), nase (Keckeis et al. 2001) and Atlantic mackerel (Mendiola et al. 2007a) have shown there is no interaction between larval weight or length and incubation temperature during early development. It could be interesting to evaluate this phenomenon in other lepisosteid larvae to determine whether it is a specific family pattern or exclusive of the Cuban gar.
Every fish species has an optimum temperature for growth (Kooka et al. 2007) that differs even at each developmental stage (Jonassen et al. 1999, Saka et al. 2005). The typical bell-shaped curve described by Jobling (1997) shows that maximum growth rates are achieved at some middle temperature, with decreasing growth rates at lower and higher temperatures (Kling et al. 2007, Rushworth et al. 2011). Our results suggest that a lack of data, particularly for the lower temperatures, has consequences in establishing the optimum temperature for growth of the Cuban gar larvae. Prior knowledge of the growth temperature relationship indicates that this species has better growth rates at 26 °C during the first 18 days after hatching (larval period). However, attempts will be made to determine its optimum temperature in order to optimize the culture conditions for this stage. Studies on A. spatula and A. tropicus showed the best growth at 30°C (Márquez 1998, Aguilera et al. 2002), in contrast with our results.
Comabella et al. (2013b) provided a detailed explanation of the allometric growth of this species using larvae reared at 28°C. The present analysis revealed that some body proportions changed considerably during larval development depending on the incubation temperature. In relation to larval weight growth, it was interesting to find the same negatively allometric growth, with a similar growth coefficient and identical inflexion points for the three incubation temperatures. However, many morphological characters such as the head, snout, paired fins, trunk (lengths), head and pectorals (heights), showed a remarkable anticipation of the inflexion point with an increase in incubation temperature.
Many fish species exhibit allometric growth during the larval period, from the absorption of the yolk sac to the onset of metamorphosis (Mello et al. 2006, Geerinckx et al. 2008). These patterns reflect morpho-anatomical growth priorities according to their importance for primary living functions (Sala et al. 2005, Choo Liew 2006) that guarantee an appropriate survival. As mentioned above, increasing temperatures accelerated not only many inflexion points but also the morphological development stages. Yolk depletion varied from 13 to 8 d when water temperature increased from 26 to 30°C, indicating that the rate of yolk use was directly proportional to the temperature. However, the significant change in larval wet weight occurred at 14 DAH for the three temperatures. Although the final survival rate under different temperature conditions was similar, the least gain in length and weight in the exotrophic phase was obtained at 30°C. These results indicate that a faster larval development at high temperatures seems to be disadvantageous as an early strategy. The early life stages of Cuban gar are considered to have survival strategies characterized by piscivorous feeding habits, precocious digestive systems (Comabella et al. 2006, Comabella et al. 2013a) and fast growth rates (Comabella et al. 2010). However, the absorption of the endogenous nutrition within a few days when at 30°C left the larvae with only a short period to change from endogenous to exogenous nutrition, a process that involves various complex morphological and physiological changes.
The results of the present laboratory study constitute the first available data on the development, growth and survival of Cuban gar larvae reared at different incubation temperatures. Although it was not possible to establish their optimal temperature, the best hatching success and larval growth was recorded at 26°C. Also, a temperature of 30 °C shortened the incubation period and caused an earlier onset of exogenous feeding, it affected larval survival at the time of hatching and reduced weight and length gain during the exotrophic stage.
ACKNOWLEDGEMENTS
This study was supported by the Centro de Investigaciones Marinas (CIM) and the Centro de Reproducción de la Ictiofauna Indígena, in Cuba.
LITERATURE CITED
Abbink W, Blanco A, Roques J, Partridge GJ, Kloet K, Schneider O (2012) The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 330-333: 130-135.
Aguilera C, Mendoza R, Rodríguez G, Márquez G (2002) Morphological description of alligator gar and tropical gar larvae, with an emphasis on growth indicators. Transactions of the American Fisheries Society 131: 899-909.
Ahmad SM, Shah FA, Bhat FA, Bhat JIA, Balkhi MH (2011) Thermal adaptability and disease association in common carp (Cyprinus carpió communis) acclimated to different (four) temperatures. Journal of Thermal Biology 36: 492-497.
Bailey J, Alanärä A (2006) Effect of feed portion size on growth of rainbow trout, Oncorhynchus mykiss (Walbaum), reared at different temperatures. Aquaculture 253: 728-730.
Balon EK (1985) The theory of saltatory ontogeny and life history models revisited. En: Balon (Ed.), Early Life History of Fish. Junk Publishers, pp. 13-30.
Barnes R, King H, Carter CG (2011) Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture 318: 397-401.
Carey GR, Kraft PG, Cramp RL, Franklin CE (2008) Effect of incubation temperature on muscle growth of barramundi Lates calcarifer at hatch and post-exogenous feeding. Journal of Fish Biology 74: 77-89.
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García-Galano T (2006) Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiology and Biochemistry 32: 147-157.
Comabella Y, Hurtado A, García-Galano T (2010) Morphological and morphometric description of Cuban gar (Atractosteus tristoechus) larvae. Zoological Science 27: 931-938.
Comabella Y, Hernández A, Hurtado A, Canabal J, García-Galano T (2013a) Ontogenetic development of the digestive tract in Cuban gar (Atractosteus tristoechus) larvae. Review in Fish Biology and Fisheries 23(2): 245-260.
Comabella Y, Azanza J, Hurtado A, Canabal J, García-Galano T (2013b) Allometric growth in Cuban gar (Atractosteus tristoechus) larvae. Universidad y Ciencia 29(3): 301-315.
Coughlin DJ (1991) Ontogeny of feeding behaviour of first-feeding Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Science 48: 1896-1904.
Choo CK, Liew HC (2006) Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. Journal of Fish Biology 69: 426-445.
Das T, Pal AK, Chakraborty SK, Manush SM, Dalvi RS, Apte SK, Sahu NP (2009) Biochemical and stress responses of rohu Labeo rohita and mrigal Cirrhinus mrigala in relation to acclimation temperatures. Journal of Fish Biology 74: 1487-1498.
Dülger N, Kumlu M, Türkmen S, Ölçülü A, EroldoğanO, Asuman H, Öçal N (2012) Thermal tolerance of European sea bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. Journal of Thermal Biology 37: 79-82.
Frisk M, Vilhelm P, Fleng J (2012) Thermal optimum for pikeperch (Sander lucioperca) and the use of ventilation frequency as a predictor of metabolic rate. Aquaculture 324-325, 151-157.
Fuiman LA (1983) Growth gradients in fish larvae. Journal of Fish Biology 23: 117-123.
Geerinckx T, Verhaegen Y, Adriaens D (2008) Ontogenetic allometries and shape changes in the suckermouth armoured catfish Ancistrus cf. triradiatus Eigenmann (Loricariidae, Siluriformes), related to suckermouth attachment and yolk-sac size. Journal of Fish Biology 72: 803-814.
Georgakopoulou E, Katharios P, Divanach P, Koumoundouros G (2010) Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 308: 13-19.
Georgakopoulou E, Sfakianakis DG, Kouttouki S, Divanach P, Kentouri M, Koumoundouros G (2007) The influence of temperature during early life on phenotypic expression at later ontogenetic stages in sea bass. Journal of Fish Biology 70: 278-291.
Gisbert E, Sarasquete MC, Williot P, Castelló-Orvay F (1999) Histochemistry of the development of the digestive system of Siberian sturgeon during early ontogeny. Journal of Fish Biology 55: 596-616.
Gisbert E, Merino G, Muguet JB, Bush D, Piedrahita RH, Conklin DE (2002) Morphological development and allometric growth patterns in hatchery-reared California halibut larvae. Journal of Fish Biology 61: 1217-1229.
Hanna SK, Haukenes AH, Foy RJ, Buck CL (2008) Temperature effects on metabolic rate, swimming performance and condition of Pacific cod Gadus macrocephalus Tilesius. Journal of Fish Biology 72: 1068-1078.
Hart PR, Purser GJ (1995) Effects of salinity and temperature on eggs and yolk sac larvae of the greenback flounder (Rhombosolea tapirina Giinther, 1862). Aquaculture 136: 221-230.
Huang G, Wei L, Zhang X, Gao T (2008) Compensatory growth of juvenile brown flounder Paralichthys olivaceus (Temminck and Schlegel) following thermal manipulation. Journal of Fish Biology 72: 2534-2542.
Imsland AK, Sunde LM, Folkvord A, Stefansson SO (1996) The interaction of temperature and fish size on growth of juvenile turbot. Journal of Fish Biology 49: 926-940.
Imsland AK, Foss A, Koedijk R, Folkvord A, Stefansson SO, Jonassen TM (2007) Persistent growth effects of temperature and photoperiod in Atlantic cod Gadus morhua. Journal of Fish Biology 71: 1371-1382.
Jobling M (1997) Temperature and growth: modulation of growth rate via temperature change. En: Wood CM, McDonald DG (Eds), Global Warming: Implications for Freshwater and Marine Fish. Cambridge University Press, pp. 223-254.
Jonassen TM, Imsland AK, Stefansson SO (1999) The interaction of temperature and fish size on growth of juvenile halibut. Journal of Fish Biology 54: 556-572.
Kamler E, Szlaminska M, Kuczynski M, Hamácková J, Kouril J, Dabrowski R (1994) Temperature-induced changes of early development and yolk utilization in the African catfish Ciarías gariepinus. Journal of Fish Biology 44: 311-326.
Keckeis H, Kamler E, Bauer E, Schneeweiss K (2001) Survival, development and food energy partitioning of nase larvae and early juveniles at different temperatures. Journal of Fish Biology 59: 45-61.
Kling LJ, Hansen JM, Jordaan A (2007) Growth, survival and feed efficiency for post-metamorphosed Atlantic cod (Gadus morhua) reared at different temperatures. Aquaculture 262: 281-288.
Kooka K, Yamamura O, Nishimura A, Hamatsu T, Yanagimoto T (2007) Optimum temperature for growth of juvenile walleye pollock Theragra chalcogramma. Journal of Experimental Marine Biology and Ecology 347: 69-76.
Le Y, Sheng-Yun Y, Xiao-Ming Z, Min L, Jing-Yi L, Kai-Chang W (2011) Effects of temperature on survival, development, growth and feeding of larvae of yellowtail clownfish Amphiprion clarkii (Pisces: Perciformes). Acta Ecológica Sinica 31: 241-245.
León R, Aguiar R, Hernández I (1978) Estudio sobre la biología y el cultivo artificial del manjuarí (Atractosteus tristoechus) Blosh y Schneider. Investigación 85. Dirección Ramal de Acuicultura. La Habana, 25 p.
Loffler J, Ott A, Ahnelt H, Keckeis H (2008) Early development of the skull of Sander lucioperca (L.) (Teleostei: Percidae) related to growth and mortality. Journal of Fish Biology 72: 233-258.
Makrakis MC, Nakatani K, Bialetzki A, Sanches PV, Baumgartnera G, Gomes LC (2005) Ontogenetic shifts in digestive tract morphology and diet of fish larvae of the Itaipu Reservoir, Brazil. Environmental Biology of Fishes 72: 99-107.
Márquez H (1998) Efectos de la temperatura en el desarrollo de embriones y el crecimiento de larvas de pejelagarto Atractosteus tropicus bajo condiciones de laboratorio. División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, Tabasco, 38 p.
Martin P, Rancon J, Segura G, Laffont J, Boeuf G, Dufour S (2011) Experimental study of the influence of photoperiod and temperature on the swimming behaviour of hatchery-reared Atlantic salmon (Salmo salar L.) smolts. Aquaculture http://dx.doi.org/10.1016/j.aquaculture.2011.ll.047
Martins ML, Xu DH, Shoemaker CA, Klesius PH (2011) Temperature effects on immune response and hematological parameters of channel catfish Ictalurus punctatus vaccinated with live theronts of Ichthyoph-thirius multifiliis. Fish and Shellfish Immunology 31: 774-780.
Mello FT, Iglesias C, Borthagaray Al, Mazzeo N, Vilches J, Larrea D, Ballabio R (2006) Ontogenetic allometric coefficient changes: implications of diet shift and morphometric traits in Hoplias malabaricus (Bloch) (Characiforme, Erythrinidae). Journal of Fish Biology 69: 1770-1778.
Mendiola D, Ibaibarriaga L, Alvarez P (2007a) Thermal effects on growth and time to starvation during the yolk-sac larval period of Atlantic mackerel Scomber scombrus L. Journal of Fish Biology 70: 895-910.
Mendiola D, Alvarez P, Cotano U, Murguia AM (2007b) Early development and growth of the laboratory reared north-east Atlantic mackerel Scomber scombrus L. Journal of Fish Biology 70: 911-933.
Mendoza R, Aguilera C, Rodríguez G, González M, Castro R (2002) Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Fish Biology and Fisheries 12: 133-142.
Morales G (1999) Especies nativas de México. Acuavisión 12(2): 7-9.
Moteki M, Yoseda K, Sahin T, Ustundag C, Kohno H (2001) Transition from endogenous to exogenous nutritional sources in larval Black Sea turbot Psetta maxima. Fisheries Science 67: 571-578.
Nerici C, Merino G, Silva A (2012a) Effects of two temperatures on the oxygen consumption rates of Seriolella violácea (palm fish) juveniles under rearing conditions. Aquacultural Engineering 48: 40-46.
Nerici C, Silva A, Merino G (2012b) Effect of two temperatures on ammonia excretion rates of Seriolella violácea (palm fish) juveniles under rearing conditions. Aquacultural Engineering 46: 47-52.
Ojanguren AF, Reyes-Gavilán FG, Rodríguez R (1999) Effects of temperature on growth and efficiency of yolk utilisation in eggs and pre-feeding larval stages of Atlantic salmon. Aquaculture International 7: 81-87.
Osse JWM, Boogart JGM (2004) Allometric growth in fish larvae: timing and function. American Fisheries Society Symposium 40: 167-194.
Pérez E, Matamoros Y, Ellis S (1999) Taller para el análisis de la conservación y manejo planificado de una selección de especies cubanas (CAMP). Sección IV, Peces, Habana.
Petry AC, Agostinho AA, Piaña PA, Gomes LC (2007) Effects of temperature on prey consumption and growth in mass of juvenile trahira Hoplias aff. malabaricus (Bloch, 1794). Journal of Fish Biology 70: 1855-1864.
Rahn H, Rahn KB, Howell BJ, Gans C, Tenney SM (1971) Air breathing of the garfish (Lepisosteus osseus). Respiration Physiology 11: 285-307.
Rana KJ (1990a) Influence of incubation temperature on Oreochromis niloticus (L.) eggs and fry I. gross embryology, temperature tolerance and rates of embryonic development. Aquaculture 87: 165-181.
Rana KJ (1990b) Influence of incubation temperature on Oreochromis niloticus (L.) eggs and fry II. survival, growth and feeding of fry developing solely on their yolk reserves. Aquaculture 87: 183-195.
Reber CM, Bennett WA (2007) The influence of thermal parameters on the acclimation responses of pinfish Lagodon rhomboides exposed to static and decreasing low temperatures. Journal of Fish Biology 71: 833-841.
Rushworth K, Smith S, Cowden K, Purcell S (2011) Optimal temperature for growth and condition of an endemic subtropical anemone fish. Aquaculture 318: 479-482.
Ruyet JP, Buchet V, Vincent B, Delliou HL, Quemener L (2006) Effects of temperature on the growth of pollack (Pollachius pollachius) juveniles. Aquaculture 251: 340-345.
Saka ?, Firat K, Okan H, BLike E (2005) The effect of temperature on embryonic development of the Red porgy (Pagrus pagrus) eggs. E.U. Journal of Fisheries and Aquatic Sciences 22: 95-99.
Sala R, Santamaria CA, Crespo S (2005) Growth of organ systems of Dentex dentex (L) and Psetta maxima (L) during larval development. Journal of Fish Biology 66: 315-326.
Seikai T, Tanangonan JB, Tanaka M (1986) Temperature influence on larval growth and metamorphosis of the Japanesse flounder Palalichthys olivaceus in the laboratory. Bulletin of the Japanesse Society for the Science of Fish 52: 977-982.
Simon T, Wallus R (1989) Contributions to the early life histories of gar (Actinopterygii: Lepisosteidae) in the Ohio and Tennesse river basins with emphasis on larval development. Transactions of the Kentucky Academy of Science 50: 59-74.
Smatresk NJ, Cameron JN (1982a) Respiration and acid-base physiology of the spotted gar, a bimodal breather II. Responses to temperature change and hypercapnia. Journal of Experimental Biology 96: 281-293.
Smatresk NJ, Cameron JN (1982b) Respiration and acid-base physiology of the spotted gar, a bimodal breather I. Normal values and the response to severe hypoxia. Journal of Experimental Biology 96: 263-280.
Snik GMJ, Boogaart JGM, Osse JWM (1997) Larval growth patterns in Cyprinus carpio and Clariasgariepinus with attention to the finfold. Journal of Fish Biology 50: 1339-1352.
Steinarsson A, Björnsson B (1999) The effects of temperature and size on growth and mortality of cod larvae. Journal of Fish Biology 55 (Supplement A): 100-109.
Sun L, Chen H, Huang L (2006) Effect of temperature on growth and energy budget of juvenile cobia (Rachycentron canadum). Aquaculture 261: 872-878.
Teletchea F, Gardeur J-N, Kamler E, Fontaine P (2009) The relationship of oocyte diameter and incubation temperature to incubation time in temperate freshwater fish species. Journal of Fish Biology 74: 652-668.
Wang YL, Buddington RK, Doroshov SI (1987) Influence of temperature on yolk utilization by the white sturgeon, Acipenser transmontanus. Journal of Fish Biology 30: 263-271.
Williams K, Papanikos N, Phelps RP, Shardo JD (2004) Development, growth and yolk utilization of hatchery-reared red snapper Lutjanus campechanus larvae. Marine Ecology Progress Series 275: 231-239.