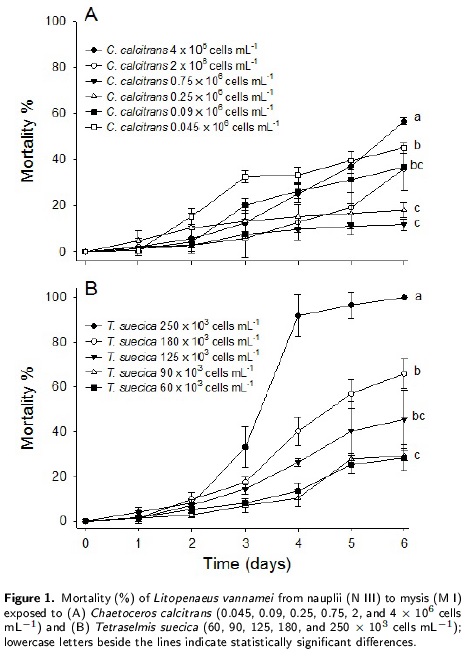
Larval shrimp mortality in relation to algal food density
Changes in mortality rates during the larval stage of the Pacific white shrimp (Litopenaeus vannamei) on the basis of algal (Chaetoceros calcitrans or Tetraselmis suecica) food density
Cambios en las tasas de mortalidad durante el estadio larval del camarón blanco del Pacífico (Litopenaeus vannamei) en base a la densidad de alimento algal (Chaetoceros calcitrans o Tetraselmis suecica)
Alfredo Pérez-Mora les1,2*, Christine J. Band-S chmidt2, Sergio F. Martínez-Díaz2
1Centro Universitario de Investigaciones Oceanológicas, Universidad de Colima. Carretera Manzanillo-Barra de Navidad Km 20, Col. El Naranjo. CP. 28860. Manzanillo, Colima, México.
2Centro Interdisciplinary de Ciencias Marinas, Instituto Politécnico Nacional, Departamento de Plancton y Ecología Marina. Av. IPN s/n, CP. 23096. La Paz, Baja California Sur, México
*Autor de correspondencia: alfredperezmorales@gmail.com
The received february 03, 2015
accepted January 07, 2016
ABSTRACT
In the shrimp culture, larval stages have high mortality rates, particularly in the zoea stage, this because to the star of phytoplankton feeding and an inadequate microalga species and cell density used in commercial hatcheries. Several microalgae species are used as food in shrimp larviculture, and the most common are Chaetoceros calcitrans and Tetraselmis suecica. Therefore, in this study, we quantified changes in the mortality rates of zoea larvae of L. vannamei fed either C. calcitrans or T. suecica at different cell densities. Results showed higher mortality rates when fed L. vannamei larvae with T. suecica than C. calcitrans. This study demonstrates that when zoea begin to feed on phytoplankton, they are highly sensitive to the microalga diet and cell densities supplied, which significantly affect the survival of L. vannamei larvae.
Key words: Aquaculture, microalgae, shrimp larvae, zoea stage, zooplankton
RESUMEN
En el cultivo de camarón, los estadios larvales presentan altas mortalidades, particularmente en el estadio de zoea, debido al inicio de la alimentación fitoplanctófaga y principalmente por una inadecuada selección de la especie de microalga y de densidad celular suministrada como alimento en laboratorios de producción larvaria. Diversas microalgas son usadas como alimento en la larvicultura del camarón; las más comunes son Chaetoceros calcitrans y Tetraselmis suecica. En el presente trabajo se cuantificaron los cambios en las tasas de mortalidad de larvas en estadio de zoea de L. vannamei alimentadas con C. calcitrans o T. suecica a diferentes densidades celulares. Los resultados mostraron mayores mortalidades en larvas de L. vannamei cuando se alimentaron con T. suecica en comparación con C. calcitrans. Este estudio demostró que cuando las larvas en zoea comienzan a alimentarse de fitoplancton, son altamente sensibles al tipo de microalga y a la densidad celular suministrada, lo cual afecta significativamente la sobrevivencia de larvas de L. vannamei.
Palabras clave: Acuacultura, estadio de zoea, larvas de camarón, microalgas, zooplancton
INTRODUCTION
In Mexico, shrimp aquaculture is a highly profitable industry widely developed in the states of Sinaloa, Sonora, and Nayarit. The main farm-raised species in these regions is the Pacific white shrimp Litopenaeus vannamei (Boone 1931), for which have been developed intensive breeding and rearing techniques from the post-larval to adult stages. However, the larval stages of zoea have the highest mortalities in the shrimp life cycle, predominantly because to an inadequate selection of microalgae species and cell densities used as food in commercial hatcheries (Godinez et al. 2005, Pina et al. 2006). Microalgae are widely used as the main source of food for larvae of farmed crustaceans, although several characteristics need to be considered, such as biochemical composition (fatty acids, amino acids, vitamins, and enzymes), digestibility, size, and shape, examples of suitable microalgae are Isochrysis, Chaetoceros, Thalassiosira, Tetraselmis, Skeletonema, Chlorella, and Dunaliella (Volkman et al. 1989, Brown et al. 1999, Nunez et al. 2002, Hemaiswarya et al. 2011).
Microalgae commonly used in hatcheries of Mexico for routine feeding protocols are Chaetoceros calcitrans and Tetraselmis suecica (Perez-Morales et al. 2015); these microalgae are supplied without quantifying the cell density to feed shrimp larvae, which may cause high mortalities in the zoea stage (Godinez et al. 2005, Pina et al. 2006). Therefore, in this study, we quantified changes in the mortality rates of zoea of L. vannamei fed either C. calcitrans or T. suecica at different cell densities.
MATERIAL AND METHODS
Strains of microalgae used as the food source, C. calcitrans (8-12 µm) and T. suecica (30-35 µm) were grown at 23 ± 1 °C, 37 ± 1 ups, pH 8.0 ± 0.1, photoperiod of 12:12 h light:dark cycle, and artificial illumination of 150 µmol m-2 s-1 in 2-L bottles with gentle air influx and semi-continuous cultures using the f/2 medium; silicates were added for C. calcitrans and not added for T. suecica (Perez-Morales et al. 2015). Both strains were cultured for several weeks prior to the bioassays. Cell density was estimated by direct counting with a Neubauer hemocytometer (Hausser Scientific); seawater previously autoclaved and filtered under a low vacuum with Whatman GF/F filters was used for dilutions.
Three batches of L. vannamei nauplii were donated by a local hatchery. In the laboratory, the three batches were mixed to eliminate the effect caused by progeny. Stage III nauplii (N III) were maintained in darkness at room temperature (26 °C). Nauplii vitality was verified by observing at traction to light. All nauplii that swam vigorously towards the light source were considered good candidates for the bioassay. These nauplii were incubated by adding one nauplius per well to 48 polystyrene microdilution well-plates with sterile Pasteur pipettes. In each well, 1 mL of different cell densities of C. calcitrans (0.045, 0.09, 0.25, 0.75, 2, and 4 x 106 cells mL-1 ) or T. suecica (60, 90, 125, 180, and 250 x 103 cells mL-1) was added. Each set of nauplii was incubated in triplicate at 23 °C and 37 ups of salinity in an acrylic incubator. Our experimental design was as follows: for C. calcitrans, we used 864 nauplii (6 cell densities x 3 replicates with 48 nauplii in each plate), and for T. suecica, we used 720 nauplii (5 cell densities x 3 replicates with 48 nauplii in each plate). In total, 33 polystyrene microdilution well-plates were used.
The plates were observed every day, and the survival of L. vannamei was quantified. The bioassay ended when nauplii reached the mysis stage (M I). Identification of the early development stages of L. vannamei was based on the descriptions reported by Kitani (1986). To find differences among treatments, mortality data for each microalga species used as food were statistically tested using two-way statistical analyses of variance (ANOVA, p < 0.05); for multiple comparisons, Tukey’s post-hoc tests were performed (Sokal and Rohlf 1981).
RESULTS AND DISCUSSION
At the beginning of day three, the stage I zoea was observed, while stage I mysis was found at the end of day six in both microalgal bioassays. These results showed different mortality rates for zoea stages depending on the microalga supplied as feed. The C. calcitrans bioassay showed the highest mortality (~60 %) for the zoea stage at a cell density of 4 x 106 cells mL-1(figure 1A) a cell densities of 2, 0.09 and 0.045 x 10-6cells mL-1 showed mortalities close to 40 %. A lower mortality rate (~15 %) was observed at cell densities of 0.25 and 0.75 x 106 cells mL-1. In the T. suecica bioassay, mortality rate of zoea increased from 4 % to 92 % after day two until day four at a cell density of 250 x 103 cells mL-1 (Figure 1B), and then a slight increase was observed until the end of the bioassay (100 % mortality). A lower mortality rate (~25 %) was observed at cell densities of 60 and 90 x 103 cells mL-1. These results showed statistically significant differences (p < 0.05).
Mortality rates were higher when larvae of L. vannamei were fed with T. suecica than with C. caldtrans. Similar results have been observed in other shrimp species such as Penaeus japonicus, Penaeus semisulcatus, and Penaeus monodon, in which a lower mortality rate was observed when the larvae were fed Chaetoceros muelleri than T. suecica (D’Souza and Loneragan 1999). Pina et al. (2006) reported higher survival and growth rates, and longer organisms when C. muelleri was used as food in comparison with T. suecica or Isochrysis galabana at densities from 0.1 to 0.2 x 106 cells >mL-1; they observed 100 % mortality when stage II zoea of L. vannamei were fed only T. suecica. In contrast, Loya-Javellana (1989) reported a survival rate between 85 % and 100 % in P. monodon fed Tetraselmis sp. at densities from 2.5 to 17.5 x 103 cells ml_-1. Our results for C. calcitrans differ from those reported by Godinez et al. (2005) for Litopenaeus stylirostris. They tested the effects of several cell densities of C. calcitrans on the survival of zoea stages and found that 0.09 x 106 cells ml_-1 was the best cell density for reducing shrimp mortality. For L. vannamei in this study, the optimum range was from 0.25 to 0.75 x 106 cell mL -1.
Microalgae are the main source of proteins, lipids, and carbohydrates for shrimp larvae in the zoea stage (Pérez-Morales et al. 2015); the most important nutrients that affect the performance of shrimp larvae are polyunsaturated fatty acids such as eicosapentaenoic acid (20:5ω3 [EPA]) and arachi-donic acid (20:4ω6 [ARA]) (D’Souza and Loneragan 1999, Palacios et al. 2002). Nutritional differences among diatoms and chlorophytes have been reported: in C. calcitrans, protein content is 43.1 %; lipids, 11.7 %; and carbohydrates, 6.6 %; in T. suecica, protein content is 42.6 %; lipids, 19 %; and carbohydrates, 21.7 % (% of dry weight) (Banerjee et al. 2011, Abiusi et al. 2014). Differences in the fatty acid composition have also been found: C. calcitrans has higher quantities of EPA and ARA (~11 and 5.7 %, respectively) than T. suecica (~4.8 and 1.8 %, respectively) (Volkman et al. 1989, Abiusi et al. 2014). With respect to vitamin content, Brown et al. (1999) have reported that Chaeto-ceros muelleri has a higher proportion (~125 µg g-1) of thiamine (B1) than tetraselmis sp. (~109 µg g-1). Nevertheless, Tetraselmis sp. exhibits a higher content of other essential vitamins (retinol (A), cobalamin (B12), and ascorbic acid (C); 2.2, 1.95, and 3 000 µg g-1, respectively) than other microalgae commonly used in shrimp aquaculture. EPA and ARA are commonly detected in higher quantities in C. calcitrans than in T. suecica (see above), which may explain, in part, the higher survival of C. calcitrans observed in this study.
The structure of the digestive tract in decapod crustacean species is similar; however, digestive responses to specific nutrients differ widely. Particularly, the digestive system in penaeid shrimp does not have a gastric mill during early larval development; thus, nutrients are assimilated using enzymes released mainly from the anterior midgut diverticulum and, to a lesser extent, by the hepatopan-creas (Ceccaldi 1989, Kumlu 1999). Hence, activities of digestive enzymes in L. vannamei larvae could result in the assimilation of more nutrients from diatoms than chlorophytes, mainly because of intrinsic differences among microalgal groups (Pérez-Morales et al. 2015). This may explain some of the differences in the mortality rates with respect to C. calcitrans and T. suecica in this study; such has those observed in other penaeid shrimps (D’Souza and Loneragan 1999, Pina et al. 2006).
The digestive physiology of crustacean filter feeders is regulated by a direct relationship among suspended particles available as food, feeding and filtration rates, and retention time in the digestive tract (Kumlu 1999, Pérez-Morales etal. 2014). Our results indicate that cell densities less than the optimal value may be insufficient for the energy required for normal metabolism and adequate growth rate. Besides, cell densities higher than the optimal value may induce higher particle ingestion and diminish the retention time in the digestive tract, releasing intact microalgal cells without assimilation of nutrients and causing death by starvation in zoea larvae; this has been reported in the early development stages of different decapod crustaceans (Ceccaldi 1989, Loya-Javellana 1989, Kumlu 1999).
In commercial hatcheries of Mexico, a monoalgal diet as food is a very common practice for shrimp larviculture, so the appropriate microalga species and cell density are fundamental for reducing the mortality rate in the zoea stage. In conclusion, this study demonstrates that when zoea begin to feed on phytoplankton, they are highly sensitive to the microalgal diet and cell densities supplied, which significantly affect the survival of L. vannamei larvae.
ACKNOWLEDGEMENTS
This research was supported by the institutional project SIP 2016-1180, and by the Consejo Nacional de Ciencia y Tecnologia (CONACYT) project SEP 178227. A. Pérez-Morales thanks the CONACYT for the postdoctoral fellowship granted, corresponding to Estancias Posdoctorales Vinculadas al Fortalecimiento de la Calidad del Posgrado Nacional 2014. C.J. Band-Schmidt and S.F. Martínez-Díaz are COFFA-IPN and EDI-IPN fellows. Microalgae strains were obtained from the Culture Collection of Microalgae at the Centro Interdisciplinario de Ciencias Marinas-IPN, La Paz, B.C.S., México. Authors thank Aquacultura Mahr (laboratory production of shrimp postlarvae) for donating L. vannamei nauplii and Laura G. Flores-Montijo for technical assistance.
LITERATURE CITED
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D’Ottavio M, et al. (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M-M33 grown with leds of different colors. Biotechnology and Bioengineering 11: 956-964.
Banerjee S, Hew WE, Khatoon H, Shariff M, Yusoff FMd (2011) Growth and proximate composition of tropical marine Chaetoceros calcitrans and Nannochloropsis oculata cultured outdoors and under laboratory conditions. African Journal of Biotechnology 10: 1375-1383.
Brown MR, Mular M, Miller I, Farmer C, Trenerry C (1999) The vitamin content of microalgae used in aquaculture. Journal of Applied Phycology 11: 247-255.
Ceccaldi HJ (1989) Anatomy and physiology of digestive tract of crustaceans decapods reared in aquaculture, In: Advances in tropical aquaculture. Tahiti. AQUACOP, IFREMER, Actes de Colloque 9. pp: 243-259.
D’Souza FM, Loneragan NR (1999) Effects of monospecific and mixed-algae diets on survival, development and fatty acid composition of penaeid prawn (Penaeus sp.) larvae. Marine Biology 133: 621-633.
Godinez DE, Diaz AH, Gallo MC (2005) índice de desarrollo y supervivencia de larvas del camarón azul Litopenaeus stylirostris (Stimpson, 1871), alimentadas con diferentes concentraciones de Chaetoceros calcitrans (Paulsen). Revista Colombiana de Ciencias Pecuarias 18: 27-33.
Hemaiswarya S, Raja R, Kumar RR, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World Journal of Microbiology and Biotechnology 27: 1737-1746.
Kitani H (1986) Larval development of the white shrimp Penaeus vannamei Boone reared in the laboratory and the statistical observation of its naupliar stages. Bulletin of Japanese Society of Science and Fisheries 52: 1131-1139.
Kumlu M (1999) Feeding and digestion in larval decapod crustaceans. Turkish Journal of Biology 23: 215229.
Loya-Javellana GN (1989) Ingestion saturation and growth responses of Penaeus monodon larvae to food density. Aquaculture 81: 329-336.
Núñez M, Lodeiros C, De Donato M, Graziani C (2002) Evaluation of microalgae diets for Litopenaeus vannamei larvae using a simple protocol. Aquaculture International 10: 177-187.
Palacios E, Racotta IS, Heras H, Marty Y, Moal J, Samain JF (2002) Relation between lipid and fatty acid composition of eggs and larval survival in white pacific shrimp (Penaeus vannamei, Boone, 1931). Aquaculture International 9: 531 - 543.
Pérez-Morales A, Sarma SSS, Nandini S (2014) Feeding and filtration rates of zooplankton (rotifers and cladocerans) fed toxic cyanobacterium (Microcystis aeruginosa). Journal of Environmental Biology 35: 1013-1020.
Pérez-Morales A, Martinez-Lopez A, Camalich-Carpizo JM (2015) Dry weight, carbon, C/N ratio, hydrogen, and chlorophyll variation during exponential growth of selected microalgae species used in aquaculture. CICIMAR Oceánides 30: 33-43.
Piña P, Voltolina D, Nieves M, Robles M (2006) Survival, development and growth of the Pacific white shrimp Litopenaeus vannamei protozoea larvae, fed with monoalgal and mixed diets. Aquaculture 253: 523-530.
Sokal RR, Rohlf FJ (1981) Biometry: The principles and practice of statistics in biological research. Freeman, WH and Company. New York, USA. 859p.
Volkman J K, Jeffrey SW, Nichols PD, Rogers Gl, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology 128: 219-240.