Nota Cíentifica
MOLECULAR SURVEY OF Ehrlichia canis IN DOGS FROM MEXICO: PREVALENCE OF INFECTION AND POSSIBLE ASSOCIATED FACTORS
Estudio molecular de Ehrlichia canis en perros de México: prevalencia de infección y posibles factores asociados
Olga Carolina Díaz-Medina1, Manuel Emilio Bolio-González1*, Roger Iván Rodríguez-Vivas1, Edwin José Gutiérrez-Ruíz1, Carlos Pérez-Osorio2
1 Cuerpo Académico de Salud Animal. Facultad de Medicina Veterinaria y Zootecnia. Campus de Ciencias Biológicas y Agropecuarias. Universidad Autónoma de Yucatán. Km 15.5 carretera Mérida-Xmatkuil. CP. 97100, Mérida, Yucatán, México. *Corresponding author: bgonza@correo.uady.mx
2 Departamento de Enfermedades Infecciosas y Parasitarias. Campus de Ciencias de la Salud. Facultad de Medicina de la Universidad Autónoma de Yucatán, México.
Nota cientifica recibida el 24 de marzo del 2015
Aceptada el 17 de septiembre de 2015
ABSTRACT
A cross-sectional study was performed to estimate the prevalence of infection of Ehrlichia canis in dogs and to explore the factors associated with the presence of the bacteria. The study was carried out in a village in Yucatan,Mexico. Blood samples were obtained from 200 dogs. Samples were analyzed by nested-PCR to detect the presenceof E. canis DNA and thrombocyte counts were calculated. One-hundred and forty of the dogs (70 %) were found tobe infested with ticks. A total of 1 116 ticks were recovered and all were identified as Rhipicephalus sanguineus. Theprevalence of E. canis infection was 69.2 %. None of the variables studied (gender, age, body condition, platelet-relatedbleeding, thrombocytopenia, and presence of ticks) showed association with E. canis infection. In conclusion, there isa high probability that dogs living in Yucatan, Mexico are infected with E canis.
Key words: Dogs, Ehrlichia canis, Prevalence, Nested-PCR, Yucatan, Mexico.
RESUMEN
Un estudio de sección cruzada fue realizada para estimar la prevalencia de infección de Ehrlichia canis en perros y explorar la presencia de factores asociados con la presencia de la bacteria. El estudio se realizó en una comunidad de Yucatán, México. Muestras de sangre de un total de 200 perros fueron obtenidas. Las muestrasfueron analizadas por Nested-PCR para detectar la presencia de ADN de E. canis y se calculó el conteo de Plaquetas.140 perros de 200 estudiados (70 %) estuvieron infestados por garrapatas. Un total de 1 116 garrapatas fueron recuperadas y todas identificadas como Rhipicephalus sanguineus. La prevalencia de infección por E canis fue de 69.2%. Ninguna de las variables estudiadas (género, edad, condición corporal, hemorragias relacionadas con plaquetas,trombocitopenia y presencia de garrapatas) mostraron asociación con la infección por E canis. En conclusión, existeuna alta probabilidad que los perros que viven en Yucatán, México, estén infectados con E canis.
Palabras clave: Perros, Ehrlichia canis, Prevalencia, PCR anidado, Yucatán, México.
INTRODUCTION
Ehrlichia canis is a tick-borne obligate intracellular Gram-negative bacterium that infects canine monocytes and is the primary etiologicalagent of Canine Monocytic Ehrlichiosis (CME). Thepathogen is transmitted to canines by the browndog tick, Rhipicephalus sanguineus. Experimentalinoculations have demonstrated an incubation period of 8-20 d in which the bacteria spread throughout the body via the mononuclear-phagocyte system(Neer and Harrus 2006). Following incubation, E.canis infection may progress into three consecutiveclinical stages: acute, sub-clinical and chronic. Thedisease is characterized by severe clinical presentation with hemorrhages, thrombocytopenia and, in some cases, bone marrow failure (Harrus et al. 1997).
The disease is distributed worldwide, but the prevalence is highest in tropical and subtropical regions where the parasite and the vector are present.Mexico is considered an endemic region for canineehrlichiosis caused by E. canis (Rodriguez-Vivas etal. 2000). Previous epidemiological surveys fromMexico indicated that the seroprevalence of E. canis in apparently healthy dogs was 8.7 % (Jiménez-Coello et al. 2009) and 44.1 % (Rodriguez-Vivas etal. 2005). Age >2 years, platelet-related bleedingand thrombocytopenia were found to be associatedwith higher prevalence of E. canis seropositivity.The actual prevalence of E. canis infection maydiffer from the seroprevalence because the presenceof serum anti-E. canis antibodies indicates previous exposure to infection and not necessarily activeinfection (Neer and Harrus 2006). However, dogswith clinical ehrlichiosis may not have identifiableantibodies in the first days after the initial infection, before the development of a detectable antibody titer (Harrus et al. 1996, Neer and Harrus2006).
At present, diagnosis using molecular procedures, such as nested-polymerase chain reaction (nested-PCR), is an alternative that has the advantages of higher sensitivity and specificity thanserological procedures (Unver et al. 2001, Mavro-mastis et al. 2006, Perez et al. 2006, Aguirre etal. 2008). A large dog population (1.5 dogs perhousehold) has been reported in the study community, representing a risk to other animals, includinghumans, if the disease is present (Ortega-Pachecoet al. 2007). It is important to know the prevalence of E. canis in rural communities of Yucatan,where the environment is favorable for the bacteriaand the vector, in order to design and implementcontrol measures for dogs. The objectives of thisstudy were to estimate the prevalence of E. canis indogs and to determine possible factors associatedwith its presence in a rural community in Yucatan,Mexico.
MATERIALS AND METHODS
Study area
The study was carried out in the rural village of Molas, Yucatan, Mexico located south of the capital city, Merida (10° 49' 01" N, 89° 37' 49" W). The climate is hot and sub-humid with summer rains. The average temperature is 26 °C, and relative humidity is 83 % with a minimum of 61 %(INEGI 2007).
Study design, study population and variables
A cross-sectional study was carried in 200 dogs between November 2009 and January 2010.Dogs were randomly selected from a populationof 568 distributed in 379 houses. None of thestudied animals had been included in vaccination or parasite control programs. Blood samples were collected by jugular venipuncture using 3 ml vacuum tubes containing the anticoagulant ethylenediaminetetraacetic acid, decanted within 30 min following sampling and stored at -20 °C until DNA was extracted.
All animals were clinically examined and variables such as gender, body condition, hemorrhages and presence of ticks were recorded. The age was given by the owner or calculated bydental examination, and the body condition scorewas assessed using a slightly modified version of themethod described by Laflamme (1997).
From each sampled dog infected by ticks, at least 5 adult ticks were collected and transported tothe Laboratory of Parasitology of the Autonomous University of Yucatan School of Veterinary Medicine(LP-UADY) for tick classification, following the keysof Rodriguez-Vivas and Cob-Galera (2005).
Platelet counting
Thrombocyte counts were calculated using the method described by Cheesbrough and McArtur (1979). Fewer than 200,000 platelets of bloodwas considered to be thrombocytopenia (Harrus et al. 1997). This procedure was carried out at theLP-UADY.
DNA extraction
DNA was extracted from 200 µl of buffy coatwith a commercial kit (QIAamp® DNA Mini and Blood from QIAGEN®), accordin to the manufacturer's instructions. This extraction was performed in a different room from the nested-PCR procedure.
Nested-PCR
Nested-PCR was performed to detect the 16S rRNA gene of E. canis, using the primers ECC5’-AGAACGAACGCTGGCGGCAAGCC-3’ and ECB5’-CGTATTACCGCGGCTGCTGGC-3’ in the first reaction and ECA5’-CAATTATTTATAGCCTC-TGGCTATAGGAAA-3’ and HE-3 5’-TATAGGTACCGTCATTATCTTCCCTAT-3 in the second reaction, based on previously published work (Murphy et al., 1998). The total volume of each reaction was 50 µl, with 10 µl of extracted DNA in 10 mM TrisHCI (pH 8.3), 0.2 mM dNTPs, 1.5 mM MgCl 50 mM KCl, 0.5 µM of each primer, and 1.25 U of Taq DNA polymerase (Qiagen). DNA of E. canis (Jake strain) purified from DH/82 cells was used for positive controls, and pure water was used for negative controls. The control used in the first reaction was also used in the second reaction. The nested-PCRwas performed in a Multigene Labnet™ thermocycler. The Taq polymerase was activated for 15 min. The initial cycling consisted of 30 cycles of denaturation for 1 min at 94 °C, followed by annealing at 55 °C for 2 min and extension at 72 °C for 2 min. For the second (nested) PCR the mixture of reagents was the same, and 5 µl of the first PCR product was used as the template. The Taq polymerase was activated for 15 min. Thermocycling was in two stages: the first consisted of three cycles with denaturation for 1 min at 94 °C, followed by annealing at 55 °C for 2 min and extension at 72 °C for 1.5 min. The second stage consisted of 37 cycles, with denaturation for 1 min at 92 °C, followed by annealing at 55 °C for 2 minand extension at 72 °C for 2 min. PCR products were analyzed by 1.5 % agarose gel electrophoresis (Apex Bioresearch Products); gels were stained withethidium bromide. All procedures were carried out at the Infectious and Parasitic Diseases Laboratory of the Autonomous University of Yucatan School of Medicine. An animal sample was considered positive when the amplicon was 389 bp in size.
Data analysis
Data were analyzed using descriptive and analytic statistics. The apparent and true prevalence and confidence interval were estimated according to the formula described by Thrusfield (2007). An initial screening of dogs infected with E. canis was performed using 2x2 contingency tables of exposure variables. All variables with p < 0.20 were analyzed using a logistic-binomial regression model offixed-effects performed using SPSS vl7.0 (Statistical Package for the Social Sciences 2008) software. For analysis of associated factors, animals infected with E. canis were considered dependent variables. Gender (male, female), age (<2 years, 2-5 years, >5 years), body condition (poor, medium, good), platelet-related bleeding (yes, no), thrombocytopenia (yes, no), and presence of ticks (yes, no) were considered independent variables.
RESULTS AND DISCUSSION
One-hundred and forty of the 200 dogs studied (70 %) were found to be infested with ticks. A total of 1 116 ticks (952 females and 164 males) were recovered and all were identified as R. sanguineus. This tick is a parasite of dogs that can occasionally parasitize other hosts, including humans. It is the most widespread tick in the world and is avector of many disease agents, some of them (e.g., Coxiella burnetii, Rickettsia conorii, R. rickettsii and E. canis) being of zoonotic concern (Dantas-Torres2010). In Yucatan, Mexico, R. sanguineus hasbeen identified as the principal tick species in dogs (Rodríguez-Vivas et al. 2010). The apparent prevalence of E. canis was 71 % (142/200). Considering the sensitivity and specificity of the test, the true prevalence was 69.2 % with a confidence interval between 63.1 % and 74.6 %. The high prevalence of E. canis infection observed in this study using the nested-PCR method is similar to that reported by other researchers in different countries around the world using the same technique: India 50 % (Lakshmanan et al. 2007), United States of America 56 % (Kordick et al. 1999), Sudan 87.2 % (Inokuma et al. 2006). However, in Brazil, Sales et al. (2015) found lower prevalence of E. canis in dogs using a nested-PCR. Some studies in Yucatan have reported lower seroprevalences (detection of circulating antibodies) than the prevalence reported in the present study. Rodriguez-Vivas et al. (2005) reported in their work, which was carried out in owned dogs from the city of Merida and using a commercial diagnostic kit, a prevalence of 44.1 %. The prevalence in that case may have been underestimated because of the 79 % sensitivity of the test, as reported by Belanger et al. (2002), whichlikely produced some false negative results. Other factors that might have influenced the observed difference are sample size and bias in the selection of study animals for the serological survey. In a study performed by Jimenez-Coello et al. (2009) using indirect immunofluorescence, which is considered there ference serological test for CME, a prevalence of 8.7 % was reported. It is important to note that this study was conducted using a closed population from the city pound; this prevalence cannot be extrapolated to any other population. When estimating prevalence, the characteristics of the diagnostic test, the method of selection of the animals, and the characteristics measured must be carefully considered. In the present study, bacterial DNA fromblood was measured. Despite the high sensitivity and specificity reported for this method (Aguirre et al. 2008), the prevalence is expected to be lower than that using a test that detects antibodies, which usually remain in the host animal longer than bacterial DNA.
The high E. canis prevalence in the community of Molas could also be due to environmental conditions that allow the completion of the life cycle of the vector R. sanguineus (70 % of the studied dogs were infected by R. sanguineus) and the agent E. canis, such as temperature, humidity, lackof vector control strategies and the wandering of dogs around the town. The possibility of the dogs coming into contact with other reservoirs of the disease (wildlife) is also present (Dantas-Torres 2010). The results in our study agree with those reported by Carvalho et al. (2008), who found a higher E. canis prevalence in dogs from a rural communitywhen compared to dogs from an urban area.
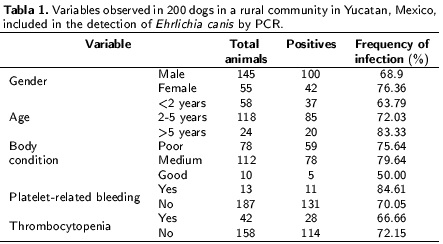
The frequencies of the studied variables are shown in Table 1. 6.5 % (13/200) of the dogs presented hemorrhages and 21.0 % (42/200) had thrombocytopenia. Thrombocytopenia and hemorrhages are the main hematological abnormalities observed in dogs with CME (Dagnone et al. 2003, Rodriguez-Vivas et al. 2005). In this study a low frequency of those two variables was observed. No epidemiological associations or statistically significant differences (p < 0.05) in gender, age, body condition, platelet-related bleeding,thrombocytopenia, or presence of ticks occurrencewere observed been dogs that were positive and negative for E. canis infection. These results differ from those reported by Bulla et al. (2004), Rodríguez-Vivas et al. (2005) and Santos et al. (2009), who found that seropositive and infected dogs had abnormal platelet counts with a statistically significant association between this variableand the presence of the disease. On the otherhand, Santos et al. (2009) reported that 46.7 % of dogs exhibiting thrombocytopenia were not infected with E. canis, indicating the probability of another etiology. A possible explanation for the low frequency of thrombocytopenia in the dogs in thepresent study is that a high percentage of the infected dogs may have been in the sub-clinical stageof the disease at the time of sampling. The samecould apply to the low observation of cutaneous andocular hemorrhages, which are usually seen duringthe acute and chronic stages of CME (Santos et al. 2009).
In the primary screening, age and body condition had p < 0.20; however, neither variable showed association with E. canis infection because their confidence interval included the unit when analyzed by logistic regression. In contrast to the report by Rodriguez-Vivas et al. (2005), no significant association between age and seropositivity was found. However, these results are consistent with those reported by Carvalho et al. (2008) who also found no significant association. The findings of the presentstudy may indicate that infection of animals with E. canis can occur at any age when the animal is incontact with the vector and the bacterium.
No statistically significant association was found between gender and the presence of E. canis, which is in agreement with previous reports by Carvalho et al. (2008), Leiva et al. (2005), and Rotondano et al. (2015). Moreover, no statistically significant association was found between body condition and the presence of E. canis, indicating that this variable probably does not influence infection. Finally, it is important to point out that one of the limitations to consider when studying associations incross-sectional epidemiological studies is that they cannot measure causality. It is not possible to determine which event occurred first, the associated factor or the disease. In conclusion, there is a high probability that dogs living in Yucatan, are infected with E. canis. It is there fore important to implement vector control measures in the community toreduce the risk of infection.
LITERATURA CITADA
Aguirre E, Tesouro M, Amusategui I, Rodríguez-Franco F, Sainz A (2008) Comparison between different polymerase chain reaction methods for the diagnosis of Ehrlichia canis infection. Annals of the NewYork Academic Sciences 1149: 118-120.
Belanger M, Sorenson HL, France MK, Bowie MV, Barbet AF, Breitschwerdt EB, et al. (2002) Comparison of serological detection methods for diagnosis of Ehrlichia canis infection in dogs. Journal of ClinicalMicrobiology 40: 3506-3508.
Bulla C, Kiomi TR, Pessoa AJ, Trinca LA, Souza LR, Wiedmeyer CE (2004) The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Veterinary Research35: 141-146.
Carvalho FS, Wenceslau AA, Carlos RS, Albuquerque GR (2008) Epidemiological and molecular study of Ehrlichia canis in dogs in Bahia, Brazil. Genetic Molecular Research 7: 657-662.
Cheesbrough M, McArtur J (1979) Manual de laboratorio para hospitales rurales en zonas tropicales. México DF: Continental 293p.
Dagnone AS, De Moráis HS, Vidotto MC, Jojima VO (2003) Ehrlichiosis in anemic, thrombocytopenic, or tick-infested dogs from a hospital population in South Brazil. Veterinary Parasitology 117: 85-290.
Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites &. Vectors 3: 1-26.
Harrus S, Wane, T, Avidar Y, Bogin E, Peh H, Bark H (1996) Serum protein alterations in canine ehrlichiosis. Veterinary Parasitology 66: 241-249.
Harrus S, Bark H, Waner T (1997) Canine monocytic ehrlichiosis: an update. Compendium Continuing Education Practicing Veterinarian 19: 431-444. INEGI (2002) Anuario estadístico del Estado de Yucatán, México. Instituto Nacional de Estadística, Geografía e Informática, México. 450p.
Inokuma H, Oyamada M, Davoust B, El Boni M, Dereure J, Bucheton B, et al. (2006) Epidemiological survey of Ehrlichia canis and related species infection in dogs in Eastern Sudan. Annals of the NewYork Academic Sciences 1071: 461-463.
Jiménez-Coello M, Pérez-Osorio C, Vado-Solis I, Rodríguez-Buenfil JC, Ortega-Pacheco A (2009) Serological survey of Ehrlichia canis in stray dogs from Yucatan, Mexico, using two different diagnostic tests.Vector-Borne Zoonotic Disease 9: 209-211.
INEGI. Instituto Nacional de Estadística y Geografía. (2007). Censo Agrícola, Ganadero y Forestal. http://www.inegi.org.mx. Fecha de consulta 23 de febrero de 2014.
Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, et al. (1999) Coinfection with multiple tick-born pathogens in a walker hound kennel in North Carolina. Journal Clinical Microbiology 37: 2631-2638.
Laflamme DP (1997) Development and validation of a body condition score system for dogs. Canine Practice 22: 10-15.
Lakshmanan B, John L, Gomathinayagam S, Dhinakarraj G (2007) Molecular detection of Ehrlichia canis from blood of naturally infected dogs in India. Veterinary Archives 77: 307-312.
Leiva M, Naranjo C, Peña T (2005) Ocular signs of canine monocytic ehrlichiosis: a retrospective study in dogs from Barcelona, Spain. Veterinary Ophthalmology 8: 387-393.
Neer TM, Harrus S (2006) Canine monocytotropic ehrlichiosis and neorickettsiosis (E. canis, E. chaffeensis, E. ruminatium, N. sennetsu and N. risticii infection). In: Greene CE (ed.) Infectious diseases of thedog and cat. St. Louis, USA, Saunders Elsevier, pp: 203-217.
Mavromastis K, Kuyler DC, Lykidis A, Ivanova N, Francino MP, Chain P, et al. (2006) The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure andimmune evasion strategies. Journal Bacteriology 188: 4015-4023.
Ortega-Pacheco A, Rodríguez-Buenfil JC, Bolio-González ME, Sauri-Arceo CH, Jiménez-Coello M, Forsberg CL (2007) A survey of dog populations in urban and rural areas of Yucatan, Mexico. Anthrozoos 20:261-274.
Pérez M, Bodor M, Zhang C, Ziong Q, Rikihisa Y (2006) Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Annals of the New York Academic Sciences 1078: 110-117.
Rodríguez-Vivas Rl, Albornoz REF, Bolio-González ME (2005) Ehrlichia canis in dog in Yucatan. Seropreva-lence, prevalence of infection and factors associated. Veterinary Parasitology 127: 75-79.
Rodriguez-Vivas Rl, Cob-Galera LA, Dominguez-Alpizar JL (2000) Hemoparásitos en bovinos, caninos y equinos diagnosticados en el laboratorio de parasitología de la Facultad de Medicina Veterinaria y Zootecnia de la Universidad Autónoma de Yucatán (1984-1999). Revista Biomédica 11: 277-282.
Rodríguez-Vivas RI, Manrique-Saide P, Ramírez-Cruz GT, Cob-Galera L, Rosado-Aguilar JA, Bolio-González M (2010) Insectos y ácaros de importancia médica y veterinaria. En: Diversidad y desarrollo humanoen Yucatán. CICY, CONABIO, SEDUMA. Mérida, México, pp: 303-305.
Rotondano TE, Almeida HK, Krawczak F S, Santana VL, Vidal IF, Labruna MB, et al. (2015) Survey of Ehrlichia canis, Babesia spp. and Hepatozoon spp. in dogs from a semiarid region of Brazil. Revista Brasileira de Parasitología Veterinária 24: 52-58.
Sales MRRP, Ignacchiti MDC, Mendes Junior AF, Suhett WG, Porfirio LC, Marins M, et al. (2015) Prevalence of Ehrlichia canis using the nested-PCR, correlation with the presence of morulae and thrombocytopenia in dogs treated in Veterinary Hospital of the Federal University of Espirito Santo. Revista Brasileira de Medicina Veterinària 37: 47-51.
Santos F, Coppede J, Pereira JA, Oliveira L, Roberto P, Benedetti R, et al. (2009) Molecular evaluation of the incidence or Erlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto. The Veterinary Journal 179:145-148.
(SPSS) Statistical Package for the Social Sciences V 17.0 (2008) SPSS Inc. Chicago, Illinois.
Thrusfield M (2007) Veterinary Epidemiology. Oxford, UK: Blacwell Publishing. 53p.
Unver H, Perez M, Orellana N, Huang H, Rikihisa Y (2001). Molecular and antigenic comparision of Ehrlichia canis isolates from dogs, ticks and human in Venezuela. Journal Clinical Microbiology 39: 2788-2793.