Scientific article
FEMINIZATION OF NILE TILAPIA Oreochromis niloticus (L.) BY DIETHYLSTILBESTROL GROWTH AND GONADOSOMATIC INDEX
Feminización de la tilapia del nilo Oreochromis niloticus (L.) mediante dietilestilbestrol. Crecimiento e índice gonadosomático
José Antonio Marín-Ramírez, Juan Pablo Alcántar-Vázquez*, Carolina Antonio-Estrada, Raúl Moreno-de la Torre, Daniel Calzada-Ruiz
Laboratorio de Acuicultura, Ciencias Agropecuarias. Universidad del Papaloapan, Av. Ferrocarril s/n, Col. Ciudad Universitaria, CP. 68400, Loma Bonita, Oaxaca.
Corresponding author: jupasoul@hotmail.com
Article received april 19, 2015
Accepted august 19, 2015
ABSTRACT
Sex-reversal by exogenous hormones is the most common technique used to generate monosex populations of Nile tilapia (Oreochromis niloticus). However, this technique has provoked a negative perception in recent years. Because of this, alternative techniques have been developed, including the production of YY males. Although the final product (for sale) is not administered hormones, the first part of this technique still requires the feminization of XY fry by use of estrogens, including some of a synthetic nature, such as diethylstilbestrol (DES), an estrogen that has shown particularly excellent results in related species. The aim of this study was to evaluate the effect of increasing concentrations of DES (100, 200, 300, and 400 mg kg-1) during the fry stage on the sex proportion, growth and gonadosomatic index (GSI) of Nile tilapia. The 400 mg kg-1 concentration was the one that produced the highest proportion of females (91 %). However, increasing the concentration of DES provided through diet does not guarantee a 100 % feminization rate. Additionally, the growth, survival and GSI, showed a significant decrease (p < 0.05) in all groups fed with DES compared to the control group. It is possible that the anabolic effect of DES observed in other species is not present in Nile tilapia.
Keywords: Anabolic effect, gonadal development, sex-reversal, survival
RESUMEN
La reversión sexual a través de hormonas exógenas es la técnica más usada para obtener poblaciones monosexo de tilapia del Nilo (Oreochromis niloticus). Sin embargo, esta técnica ha generado una percepción negativa en los últimos años. Debido a lo anterior, se han desarrollado técnicas alternativas incluyendo la producción de machos YY. Aunque con esta técnica el producto final (para vender) no recibe hormona, la primera parte de esta técnica aun requiere la feminización de alevines XY a través de estrógenos, incluyendo algunos de naturaleza sintética como el dietilestilbestrol (DES), el cual ha mostrado excelentes resultados en especies relacionadas. El objetivo del presente trabajo fue evaluar el efecto de concentraciones crecientes de DES (100, 200, 300, 400 mg kg-1) durante el periodo de alevín en la proporción de sexos, crecimiento e índice gonadosomático (IGS) de la tilapia del Nilo. La concentración de 400 mg kg-1 fue la que arrojó la mayor proporción de hembras (91 %). Sin embargo, incrementar la concentración de DES proporcionada a través de la dieta no garantiza una feminización del 100 %. Adicionalmente, el crecimiento, la supervivencia y el IGS, mostraron un descenso significativo (p < 0.05) en todos los grupos alimentados con DES, en comparación con el grupo control. Es posible que el efecto anabólico del DES observado en otras especies no este presente en la tilapia del Nilo.
Palabras clave: Desarrollo gonadal, efecto anabólico, reversión sexual, supervivencia
INTRODUCTION
The Nile tilapia Oreochromis niloticus (L.) is one of the most economically important species worldwide in finfish aquaculture (Nonglak et al. 2012). In commercial farming of Nile tilapia, reproduction during grow-out is a major problem, leading to the presence of fry and juveniles that overpopulate ponds and ultimately result in a wide range of fish sizes at harvest instead of the larger and more uniform fish expected from the original stocking (Mair et al. 1997, Tariq-Ezaz et al. 2004). Production of a monosex, all-male population in tilapia culture eliminates sex behavior and therefore uncontrolled reproduction, allowing the production of marketable-sized fish (Varadaraj 1989, Ponzoni et al. 2005). All-male tilapia show better growth than females, and for many years, the production of allmale tilapia has been recognized as the most effective technique to increase tilapia production (Mair et al. 1997, Müller and Hörstgen 2007, Nonglak et al. 2012).
Sex-reversa I by feeding fry with different hormones is the most common method used to produce all-male populations. However, the use of this method is increasingly being criticized. The accumulation of hormones in wild populations of fish in their natural environment, as well as the increasing number of consumers that are interested in environmentally friendly production techniques and do not want to eat products that have been treated with hormones (Müller and Hörstgen 2007, Nguyen et al. 2007, Leet et al. 2011), have led to the search for alternative techniques for the production of allmale tilapia populations. One viable alternative, on a commercial scale, is the production of genetically male tilapia (GMT™) based on crosses between YY males and XX females (Mair et al. 1997, Müller and Hörstgen 2007).
The initial development in the production of YY males requires the feminization of the XY genotypes during their sexually undifferentiated stage and the identification of these newly created sex reversed females (XY females) through a progeny test (Mair et al. 1997). However, unlike masculinization where it is feasible to obtain close to 100 % males by feeding the fry with different hormones, feminization of genotypically XY O. niloticus has proven to be more complicated.
Although there have been numerous published attempts to optimize feminization by varying parameters such as hormone type, hormone Concentration, treatment start time, duration of treatment and stocking density, this method still has some limitations (Rosenstein and Hulata 1994, Piferrer 2001). Development in recent years of YY male technology at the Universidad del Papaloapan has led to attempts to optimize the feminization process. One alternative for optimizing feminization in Nile tilapia is the use of the synthetic hormone diethylstilbestrol (DES). Application of DES in high concentrations has been associated with improved growth and feminization rates of 100 % in related species (Varadaraj 1989).
The present work was undertaken (1) to determine whether high proportions of sexually undifferentiated XY O. niloticus fry could be sex reversed to females using DES and (2) to describe the effect of DES on the gonadosomatic index and growth after normal sex differentiation in cultivated tilapia.
MATERIALS AND METHODS
Area of study
The present work was conducted at the Universidad del Papaloapan, located in Loma Bonita, Oaxaca, Mexico, at the following coordinates: 18° 06' N and 95° 53' W, at a height of 30 m above sea level. The weather in this area is warm and humid, with abundant rain during the summer. The mean temperature and average annual rainfall is 25 °C and 1845.2 mm, respectively (FAM 2014).
Broodstock
The Nile tilapia broodstock used in this research were produced using breeders from the Centro Acuicola de Temazcal (Oaxaca, Mexico) and the Sistema Cooperativo Integral (Veracruz, Mexico). This broodstock has been reared for a year in the aquaculture station of the Universidad del Papaloa-pan and fed twice a day with commercial pellets (32 % protein, Nutripec, Agribrands Purina, Irapuato Gto, Mexico).
Preparation of hormonal treatment
The synthetic hormone diethylstilbestrol (DES, Sigma Aldrich Chemical Co., St Louis, MO, USA) was added to commercial fish meal (< 0.35 mm, 53 % protein, 15 % lipids) using the alcohol evaporation method described by Guerrero (1975). In short, DES was dissolved in 95 % ethanol, sprayed over the food and kept overnight at room temperature to allow the alcohol to evaporate. Four levels of DES hormone-treatments were added to the food: 100, 200, 300 and 400 mg kg-1. The food for the control group was treated in exactly the same manner with the exclusion of the added hormone.
Fry production
The Nile tilapia broodstock were stocked at a ratio of 1:3 (male:female) in two 3 m diameter outdoor concrete tanks (28-30 °C) supplied with fertilized water. Fry were collected 14 d later with a fine-mesh net after 90 % of the water in the tanks had been siphoned. The recently hatched and sexually undifferentiated fry (~ 0.2 g weight and 8 mm length) were pooled, transported to a closed system and randomly divided into 15 glass aquaria of 85 L at an initial stocking density of 1 fry L-1. Each treatment was carried out in triplicate. During the period of treatment, a photoperiod of 12 L: 12 D was measured using an automatic timer, and water temperature was thermostatically controlled and adjusted at 25 ± 0.5 °C.
Experimental conditions
Fry were fed with the food supplemented with hormones for 20 d at 1 h intervals. The feed rate during the hormonal treatment was adjusted to 20 % of the total body weight. Random samples of 25 % of the fry per replicate were collected every 10 d. Mean wet weight was obtained using a digital scale (± 0.01) and total length was recorded from a digitized image using a imaging software (ImageJ version 1.36). Once the hormonal treatment was completed, the fry were fed with untreated commercial diet containing 50 % protein for 10 more days until the fry period was finished. Water temperature and dissolved oxygen were monitored daily using a multiparameter display system (YSI model 655, Yellow Springs Instrument Co., Inc., Yellow Springs, OH, USA). The aquaria were siphoned daily to remove feces and dead fry.
At the end of the fry period, all the juveniles from each treatment were counted, weighed and measured for the calculation of the survival rate and to determine the average weight and length. Juveniles were then transferred to outdoor 3 m diameter concrete tanks supplied with fertilized water and reared to sexual maturity (five months of age). During this period, the juveniles were fed six times a day with untreated commercial diet (44 and 40 % protein), followed by a commercial diet at 35 % protein three times a day and subsequently a commercial diet with 25 % protein until the end of the experiment. The water temperature and dissolved oxygen were monitored daily during all the post-treatment part of the experiment. Total length and wet weight were registered every 21 d for 60 random individuals per treatment.
Evaluation of sex ratio and gonadosomatic index
The sex of 60 fish per treatment was determined by external examination using methylene blue at 1 % to highlight the differences in the papilla structure. Each fish was classified as male or female. All fish were weighed, measured and sexed again by removing the gonad and the spermatic/ovarian ducts. Gonads were classified as ovaries or testes. The extracted gonads were weighed using a digital scale (±0.01) to calculate the gonadosomatic index (Sturm 1978) using the following formula:
GSI = [Gonad weight(g)/Fish weight(g)]x 100.
Statistics
Data were analyzed using statistical analysis software (Statistical version 7.0). Differences in total length and wet weight were analyzed using a one-way analysis of variance. The percentage of survival during the fry stage was arcsine transformed and analyzed using a one-way analysis of variance. The proportion of females identified in each treatment was tested against the 1:1 expectation using a chi square test at a probability of 0.1 % (p < 0.001). Differences in the gonadosomatic index between the different treatments were arcsine transformed and analyzed using Kruskal-Wallis nonparametric analysis.
RESULTS
Fry
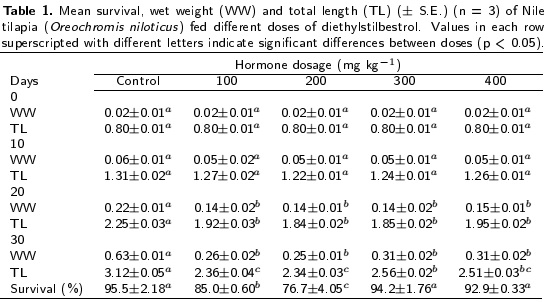
The mean wet weight and total length of the treated and control fry during the DES treatment is shown in Table 1. The wet weight and total length were significantly (p < 0.05) higher at 20 and 30 d of age in the control group than in the DES-treated groups (Table 1). In the DES-treated groups, the wet weight showed no significant differences, whereas the total length registered differences only at 30 d of age, with the concentration of 300 mg kg-1 showing a significantly (p < 0.05) larger length than that of the concentrations of 100 and 200 mg kg-1. Survival at 30 d of age was significantly lower in the concentration of 100 and 200 mg kg-1 than in the concentrations of 300 and 400 mg kg-1 and the control group. No significant differences were detected between the control group and the concentrations of 300 and 400 mg kg-1 (Table 1).
Sex-reversed females were produced in all DES treatments. The results showed that only the concentrations of 200 and 400 mg kg-1 produced progeny with a significantly higher (p < 0.001) proportion of females than the predicted 1:1 sex ratio (Table 2). The proportion of females was higher in the concentrations of 100 and 300 mg kg-1 than that observed in progeny without hormonal treatment in our laboratory (54 to 58 %); however, no significant differences were detected from normal sex ratios (Table 2). Significant differences (p < 0.05) were observed in the gonadosomatic index, with the fish of the control group showing a significantly (p < 0.05) higher value than the fish of the DES-treated groups. No significant differences were observed among the fish of the DES-treated groups (Table 2).
Survival and growth
Final survival was significantly lower (p < 0.05) in the DES-treated groups than in the control group (Table 2). The mortality in the groups fed with DES was close to 50 %, except for the concentration of 300 mg kg-1. Significant (p < 0.05) differences were observed in the post-treatment growth, with the fish of the control group being significantly heavier (Figure 1a) and larger (Figure 1b) than the fish in the DES-treated groups throughout this part of the experiment. In the groups fed with DES, the wet weight showed significant differences (p < 0.05) only at 51 and 72 d of age, with the concentration of 300 mg kg-1 showing a significantly higher value (Figure 1a) than that of the concentrations of 100 and 200 mg kg-1. As observed with the wet weight, the total length also showed significant differences (p < 0.05) only in the early days of post-treatment culture, with the concentrations of 300 and 400 mg kg-1 showing higher values than those observed in the groups treated with concen concentrations of 100 and 200 mg kg-1 at 51, 72 and 93 d of age (Figure 1b).
DISCUSSION
The aim of most of sex-reversal treatments is to control the sex phenotype in fish species of commercial interest (Devlin and Nagahama 2002, Singh et al. 2012, Singh 2013). Over the years, these treatments have provided some physiological insight as to the regulation of sex differentiation by steroid hormones (Singh 2013). Currently, it is widely accepted that endogenously synthesized steroids play an important role in the gonadal sex differentiation of many fish species. Therefore, it is possible that exogenous steroid treatments can disrupt the natural differentiation process by overriding the normal developmental pattern of gene expressions and physiological regulations leading to sex reversion, even after the initiation of the natural differentiation process (Devlin and Nagahama 2002).
Current trends in both the market and research being conducted show an interest toward decreasing the use of hormones during sex-reversal treatments that produce monosex populations for commercial purposes. One of the most important alternative techniques is the production of YY males (Mair et al. 1997). Although the first part of this technique requires the use of hormones to feminize XY fry to obtain XY females, the final product (for sale) is not administered hormones, which significantly reduces the concentration of hormones in farm effluents.
Feminization, therefore, is one of the critical stages of YY male technology. Production and identification of XY females ensures an adequate number of YY males (Mair et al. 1997). Hamdoon et al. (2013) reported an increase in the proportion of females of Nile tilapia as the concentration of DES increased. In our case, a similar increase was observed, with a higher proportion of females registered for the concentration of 400 mg kg-1 (91 %). However, as in previous reports for Nile tilapia (Potts and Phelps 1995, Hamdoon et al. 2013), 100 % females was not possible to attain using the different concentrations of DES.
The effectiveness of DES to feminize is strongly related to the biology of the particular species and the breeding techniques applied. Additionally, the interaction between genotypic factors (parental and sex determination factors) and the environment (especially temperature) could affect the rate of feminization obtained. These traits are complex and can have different degrees of interaction based on the genetic constitution and the relative strength of the sexual factors in each species (My-lonas et al. 2005).
The proportion of females observed in the concentrations of 100, 200 and 300 mg kg-1 was lower than that observed in previous reports for Nile tilapia and related species at similar concentrations (Varadaraj 1989, Hamdoon et al. 2013). This could be related to low levels of hormone uptake or the interaction with environmental variables such as water temperature. However, a genetic parental effect could also be responsible for the low proportion of females observed using these concentrations of DES. An important parental effect in Nile tilapia may be responsible, in accordance with the model of sex determination proposed by Baroiller et al. (2009), for the low proportion of females observed because of the lower parental sensitivity to feminization. It is possible that the females or males used as breeders could have displayed a genetic tendency to produce more males than females at normal rearing conditions. The low proportion of females observed in the control group (39 %) supports this. For Nile tilapia, Potts and Phelps (1995) report a proportion of females similar to that obtained in our experiment using 100 and 200 mg kg-1 of DES (> 60 %) but a lower proportion using 400 mg kg-1 of DES (80 %). On the other hand, for their study of Nile tilapia, Hamdoon et al. (2013) reported a proportion of females close to 90 % when using only 100 mg kg-1 of DES. These results support the idea that a parental genetic effect could be responsible for the proportion of females obtained due to a lower sensitivity to the hormonal treatment.
Although water temperature during hormonal treatments has been suggested as a factor responsible for deviations from expected sex ratios (Wang and Tsai 2000), in this experiment, water temperature during the fry stage (25 °C) was lower than that normally used in our laboratory (28-29 °C). Therefore, it is unlikely that the temperature could have influenced the proportion of females observed. Previous reports in Nile tilapia (Wessels and HorstgenSchwark 2011, Alcantar-Vazquez et al. 2014) have shown a significant deviation from the expected 1:1 sex ratio in both control and treated groups reared at 28 °C during a feminization or masculinization treatment.
Some natural and synthetic steroids have been shown to be growth promoters when administered at low concentrations. However, a decrease in growth induced by exposure to synthetic steroids has also been reported in several species of teleost, especially at high concentrations (Blazquez 2001, Piferrer 2001). The reduction in growth observed in the groups fed with DES compared to the control group is consistent with this. Although no significant differences were observed between the DES-treated groups, final weight and length were higher in the groups exposed to the lowest concentration of DES. A similar reduction in growth was observed by Hamdoon et al. (2013) after feeding fry of Nile tilapia with different concentrations of DES for 40 d.
In our case, the decrease in growth compared to the control group was observed in all groups fed with DES despite the increase in DES concentration. These results do not agree with those reported by Varadaraj (1989) in the Mozambique tilapia (O. mossambicus). In this case, the growth of DES-fed juveniles increased significantly compared to that of the control group as the concentration of DES increased up until a concentration equivalent to 500 mg kg-1. This difference in growth could probably be caused by a differential physiological response to an anabolic effect of DES between species. It is possible that the anabolic effect of DES (originally observed in chickens and cattle) is present in the tilapia of Mozambique but not in the Nile tilapia. Herman and Kincaid (1988) have shown that different metabolic pathways may be responsible for this increase/decrease in growth. Additionally, Ridha and Lone (1995) reported that estrogens usually show no anabolic effect in most teleosts, so the Mozambique tilapia could be one of few species of fish responding to the anabolic effect of DES.
A decrease in growth has also been observed using DES in other freshwater species, such as the European catfish (Silurusglanis) (Krol et al. 2014). In this case, the negative effect on growth was not observed when the natural estrogen estradiol-17β was used. Similar results have been observed in our laboratory in early trials comparing estradiol-17β, 17α-ethinylestradiol and DES at the same concentrations. Currently, estradiol-17β is the estrogen that shows better growth and survival than the other two synthetic hormones but results in a lower feminization rate. The Nile tilapia is probably more susceptible to synthetic estrogens whose effect at the physiological level is more powerful.
Piferrer (2001) reports that sex-reversal through estrogens (natural or synthetic) could provoke adverse effects on survival. However, this depends on a number of factors including the type of estrogen, the concentration used, the timing and the duration of the hormonal treatment. The timing of optimum hormonal treatment is based on the timing of gonadal differentiation into ovaries and testes (Lin et al. 2012). Additionally, the sensitivity at the physiological level of the species determines the magnitude of the negative effects on survival, especially if a particular threshold is exceeded (Piferrer 2001, Lin et al. 2012). Ham-doon et al. (2013) reported a decrease in survival in Nile tilapia groups fed with DES for 40 d at two different concentrations, 50 and 100 mg kg-1, whereas Varadaraj (1989) reported no significant mortality in the Mozambique tilapia when using DES in high concentrations (equivalent to 500 and 1000 mg kg-1). In other freshwater species, Zhong et al. (2005) reported a reduction in survival in groups of Chinese minnow (Gobiocypris rarus) fed with DES at three different concentrations for 30 d. In this case, survival decreased close to 50 % as the concentration of DES increased. In our work, survival showed a similar decrease (~ 50 %) in the groups fed with DES to that of the control group; however, this reduction was high immediately after the application of lower concentrations of DES rather than decreasing gradually as the concentration of DES increased. This probably could have been caused by the inhibition of the endocrine (liver-derived) and autocrine/paracrine local insulin-like growth factor I system. Shved et al. (2009) mentions that estrogens have recently been shown to inhibit this system in several species offish. The same author reported in tilapia that exposure to 17a-ethinylestradiol during early development distinctly affected the IGF system in tilapia immune organs and that this provokes interference with the antigen presentation capacity of the immune system, leading to an altered susceptibility to infections. Probably, DES, as well as 17a-ethinylestradiol, both synthetic estrogens, can provoke an increase in mortality associated with an impaired ability to fight infections in Nile tilapia. This could explain the high mortality observed in all the groups fed with DES compared to that of the control group.
The GSI is an indicator of sexual maturity of the fish and consequently of their health and nutritional status. Recent studies have shown that based on an observed reduction of GSI as well as morphological and histological changes undergone by the gonads, continuous exposure to synthetic compounds, including hormones, can induce a decrease in gonadal development (Linderoth et al. 2006, Marchand et al. 2008, Louiz et al. 2009).
In our work, gonadal examination of the fish fed with DES at different concentrations in comparison with the control group showed a significant reduction in the values of GSI and an increase in the proportion of alterations of gonadal structure. Reduction of the size of one of the two gonads or abnormal growth in one or both gonads was observed in approximately 20 % of all fish analyzed, especially the males. Similar results and abnormalities have been reported by those using DES in other freshwater species (Zhong et al. 2005, Paul-Prasanth et al. 2011). Piferrer (2001) mentioned that the presence of these abnormalities could be caused by the administration of estrogens through diet, especially at high concentrations. Song et al. (2014) reported negative effects in the GSI and serious atrophy of the gonads in several treatments in goldfish (Carassius auratus) using individual and binary mixtures of estrogens.
The negative effect of DES in the GSI observed in our experiment could have been the result of a direct effect on tissue development and gonadal structure. Haux and Norberg (1985) and Washburn et al. (1993) reported adverse effects during development on the functioning of the liver offish treated with estrogen hormones. Because the liver and gonad work closely through the action of steroid hormones, poor development of the gonad in treated fish could have an origin in the effects of estrogens on the liver. It is probable that although the DES concentrations used did not produce all females, these concentrations can be considered high at the physiological level, provoking a negative effect on the gonadal development, especially at the morphological level and with regard to functionality. Finally, Milnes et al. (2006) reported that exposure of males to estrogens can result in the reduction of testicular growth or testicular atrophy due to testicular lesions such as fibrosis and histological alterations. This could explain the high presence of abnormalities of the gonads, especially in males fed with DES.
CONCLUSIONS
Although the feminization rate was higher than in previous reports on Nile tilapia that were administered a high concentration of DES, reduction in gonadal development represents an important obstacle to the use of this synthetic hormone in future feminization treatments. The XY females produced during feminization treatments are key players in the successful development of YY males. An XY female with impaired gonadal growth has a reduced probability of being selected as a breeder. Alternative techniques of feminization need further investigation to assess whether DES has any possibility of being used in the development of Nile tilapia YY male technology.
ACKNOWLEDGEMENTS
This project has been supported by the Programa para el Mejoramiento del Profesorado (PROMEP) of Mexico (Project; PROMEP/103.5/11/6720). We thank the work groups of the Laboratorio de Acuicultura of the Universidad del Papaloapan and the Unidad de Producción Cuenca del Tesechoacan. Special thanks to James Patrick Killough for editorial improvements.
LITERATURA CITADA
Alcántar-Vázquez JP, Moreno de la Torre R, Calzada-Ruíz D, Antonio-Estrada C (2014) Production of YY-male of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) from atypical fish. Latin American Journal of Aquatic Research 42: 644-648
Baroiller JF, D'cotta H, Bezault E, Wessels S, Hoerstgen-schwark G (2009) Tilapia sex determination: where temperature and genetics meet. Comparative Biochemistry and Physiology Part A: Molecular &. Integrative Physiology 153: 30-8.
Blázquez M, Felip A, Zanuy S, Carrillo M, Piferrer F (2001) Critical period of androgen-inducible sex differentiation in a teleost fish, the European sea bass. Journal of Fish Biology 58: 342-358.
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208: 191-364.
FAM (2014) Fuerza Aerea Mexicana. Estadística meteorológica mensual. Dirección de Servicio Meteorológico. Estación Loma Bonita, Oaxaca, México. 20p.
Guerrero R (1975) Use of androgens for the production of all-male tilapia aurea (Steindachner). Transactions of the American Fisheries Society 2: 342-348.
Hamdoon NT, Ibrahim F, Kelany AM, Hanan FE, Zayed AE (2013) Hormonal sex reversal in Oreochromis niloticus by oral administration of diethylstilbestrol. Life Science Journal 10: 123-128.
Haux C, Norberg B (1985) The influence of estradiol-17β on the liver content of protein, lipids, glycogen and nucleic acids in juvenile rainbow trout, salmo gairdneri. Comparative Biochemistry and Physiology 72: 165-172.
Herman RL, Kincaid HL (1988) Pathological effects of orally administered estradiol to rainbow trout. Aquaculture 72: 165-172.
Krol J, Poblocki W, Bockenheimer T, Hliwa P (2014) effect of diethylstilbestrol (DES) and 17ß-estradiol (E2) on growth, survival and histological structure of the internal organs in juvenile European catfish silurus glanis (I.). Aquaculture International 22: 53-62.
Leet KJ, Gall EH, Sepulveda SM (2011) A review of studies on androgen and estrogen exposure in fish early life stages: effects on gene and hormonal control of sexual differentiation. Journal of Applied Toxicology 31: 379-398.
Lin S, Benfey TJ, Martin-Robichaud DJ (2012) Hormonal sex reversal in Atlantic cod, Gadus morhua. Aquaculture 364-365: 192-197.
Linderoth M, Hansson T, Liewenborg B, Sundberg H, Noaksson E, Hanson M, et al. (2006) Basic physiological biomarkers in adult female perch (Perca fluviatilis) in a chronically polluted gradient in the Stockholm recipient (Sweden). Journal of Marine Pollution Bulletin 53: 437-450.
Louiz I, Ben-Attiab M, Ben-Hassinea O (2009) Gonadosomatic index and gonad histopathology of Gobius niger (Gobiidea, Teleost) from Bizerta lagoon (Tunisia): Evidence of reproduction disturbance. Fisheries Research 100: 266-273.
Mair GC, Abucay JS, Skibinski DF, Beardmore JA (1997) Genetic manipulation of sex ratio for the large scale production of all-male tilapia, Oreochromis niloticus. Canadian Journal of Fisheries and Aquatic Sciences 54: 396-404.
Marchand MJ, Pieterse GM, Barnhoorn IJ (2008) Preliminary results on sperm motility and testicular histology of two feral fish species, Oreochromis mossambicus and Clariasgariepinus, from a currently DDT-sprayed area, South Africa. Journal of Applied Ichthyology 24: 423-429.
Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin IV, et al. (2006) Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environmental Research 100: 3-17.
Müller BA, Hörstgen SG (2007) A YY-male Oreochromis niloticus strain developed from an exceptional mitotic gynogenetic male and growth performance testing of genetically all-male progenies. Aquaculture Research 38: 773-775.
Mylonas CC, Anezaki L, Divanach P, Zanuy S, Piferrer F (2005) Influence of rearing temperature during the larval and nursery periods on growth and sex differentiation in two Mediterranean strains of Dicentrarchus labrax. Journal of Fish Biology 67: 652-668.
Nguyen NH, Khaw L, Ponzoni RW, Hamzah A, Kamaruzzaman N (2007) Can sexual dimorphism and body shape be altered in Nile tilapia (Oreochromis niloticus) by genetic means?. Aquaculture 272: 38-46.
Nonglak P, Boonanuntanasarn S, Jangprai A, Yoshizaki G, Na-Nakorn U (2012) Pubertal effects of 17a-methyltestosterone on GH-IGF-related genes of the hypothalamic-pituitary-liver-gonadal axis and other biological parameters in male, female and sex-reversed Nile tilapia. General and Comparative Endocrinology 177: 278-292.
Paul-Prasanth B, Shibata Y, Horiguchi R, Nagahama Y (2011) Exposure to diethylstilbestrol during embryonic and larval stages of medaka fish (Oryzias latipes) leads to sex reversal in genetic males and reduced gonad weight in genetic females. Endocrinology 152: 707-717
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197: 229-281.
Ponzoni RW, Hamzah A, Tan S, Kamaruzzaman N (2005) Genetic parameters and response to selection for live weight in the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture 247: 203-210.
Potts AC, Phelps RP (1995) Use de diethylstilbestrol and ethynylestradiol to feminize nile tilapia Oreochromis niloticus (L.) in an outdoor environment. Journal of applied ichthyology 11: 111-117.
Ridha MT, Lone KP (1995) Preliminary studies on feminization and growth of Oreochromis spilurus (Gunther) by oral administration of 17a-ethynyloestradiol in sea water. Aquaculture Research 26: 475-482.
Rosenstein S, Hulata G (1994) Sex reversal in the genus Oreochromis: optimization of feminization protocol. Aquaculture and Fishery Management 25: 329-339.
Shved N, Berishvili G, Hausermann E, D’Cotta H, Baroiller JF, Eppler E (2009) Challenge with 17α-ethinylestradiol (EE2) during early development persistently impairs growth, differentiation, and local expression of IGF-I and IGF-II in immune organs of tilapia. Fish and Shellfish Immunology 26: 524-530.
Singh AK (2013) Introduction of modern endocrine techniques for the production of monosex population of fishes. General and Comparative Endocrinology 181: 146-155.
Singh R, Singh AK, Tripathi M (2012) Effect of an aromatase inhibitor analogue tamoxifen on the gonad and sex differentiation in Nile tilapia Oreochromis niloticus (Linn.) Journal of Environmental Biology 33: 799-803
Song TW, Wang JZ, Liu CH (2014) Effects of individual and binary mixtures of estrogens on male goldfish (Carassius auratus). Fish Physiology and Biochemistry 40: 1927-1935.
Sturm GM L (1978) Aspects of the biology of Scomberomorus maculatus (Mitchill) in Trinidad. Journal of Fish Biology 13: 155-172.
Tariq-Ezaz M, Myers J, Powell S, McAndrew B, Penman D (2004) Sex ratios in the progeny of androgenetic and gynogenetic YY male Nile tilapia, Oreochromis niloticus L. Aquaculture 232: 205-214.
Varadaraj K (1989) Feminization of Oreochromis mossambicus by administration of diethylstilbestrol. Aquaculture 80: 337-341.
Wang HL, Tsai LC (2000) Effects of temperature on the deformity and sex differentiation of tilapia, Oreochromis mossambicus. Journal of Experimental Zoology 286: 534-537.
Washburn BS, Krantz JS, Avery EH, Freedland RA (1993) Effects of estrogen on gluconeogenesis and related parameters in male rainbow trout. American Journal of Physiology 264: 720-725.
Wessels S, Horstgen-Schwark G (2007) Selection experiments to increase the proportion of males in Nile tilapia (Oreochromis niloticus) by means of temperature treatment. Aquaculture 272: 80-87.
Zhong X, Xu Y, Liang Y, Liao T, Wang J (2005) The Chinese rare minnow (Gobiocypris rarus) as an in vivo model for endocrine disruption in freshwater teleosts: a full life-cycle test with diethylstilbestrol. Aquatic Toxicology 71: 85-95.