Artículo científico
Potential distribution zones for soybean rust (Phakopsora pachyrhizi) in Mexico
Zonas de distribución potencial para roya de la soya (Phakopsora pachyrhizi) en México
1*Ricardo Yáñez-López, 1María Irene Hernández-Zul, 2Juan Ángel Quijano-Carranza, 3Antonio Palemón Terán-Vargas, 4Luis Pérez-Moreno, 5Gabriel Díaz-Padilla, 1Enrique Rico-García
1 Cuerpo Académico de Ingeniería de Biosistemas, Facultad de Ingeniería, Universidad Autónoma de Querétaro, Centro Universitario, Cerro de las Campanas s/n, C.P. 76010, Querétaro, México.
ryanez@hotmail.com
2 Campo Experimental Bajío, (CEBAJ-INIFAP). Km 6.5 Carretera Celaya-San Miguel de Allende. Celaya, Guanajuato, México.
3 Campo Experimental las Huastecas, Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias, Carretera Tampico-Mante Km. 55, Villa Cuauhtémoc, Tamaulipas, México.
4 Universidad de Guanajuato, Instituto de Ciencias Agrícolas, Apdo. Postal 311. Irapuato, Guanajuato, México.
5 Campo Experimental Cotaxtla. Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Km. 3.5 Carretera Xalapa-Veracruz. Colonia Ánimas. Xalapa, Veracruz. Mexico.
Artículo recibido el 18 de julio de 2014
Aceptado el 16 de febrero de 2015
ABSTRACT
Asian Soybean Rust is one of the most important soybean diseases. Since the past decade, some important soybean production areas in America, like Brazil and the United States of America, have been affected by this disease. Due to the seriousness of this threaten, in 2009, the Mexican government implemented a surveillance program based on the installation and monitoring of sentinel plots in areas planted with crops considered as susceptible hosts for this organism. In order to support the strategy to prevent the establishment of the disease in Mexico, in the present study the potential distribution of the organism was evaluated considering the following criteria: 1) the suitability of climatic conditions for soybean rust; 2) the extent of the cultivated areas with susceptible hosts, and 3) the inoculums availability. Favorable days for Asian soybean rust infection were calculated with a simple model which uses climatic variables as inputs. The model was applied to classify the agricultural areas of the country according to the probability of occurrence of favorable conditions for infection of soybean rust in the summer and winter growing seasons. The Results indicate that in the summer the greatest number of favorable days for infection occurs, mainly in Tamaulipas, Veracruz, Yucatan, Chiapas, Oaxaca, Guerrero, Michoacan, Jalisco, Nayarit, Sinaloa and Sonora. In the winter, the favorable days decrease considerably. Based on these results we conclude that the probability that the Asian soybean rust would be overwintering in Mexico is low.
Key words: Phakopsora pachyrhizi, soybean, soybean rust, risk analysis
Resumen
La roya asiática es una de las enfermedades más importantes para el cultivo de la soya. Desde la década pasada zonas productoras de soya en América en Brasil y Estados Unidos se han visto afectadas por esta enfermedad. Debido a la gravedad de esta amenaza, en el 2009 el Gobierno mexicano puso en marcha un programa de vigilancia basado en la instalación y seguimiento de parcelas centinelas en zonas con cultivos hospederos. Con el fin de mejorar la delimitación de las zonas de riesgo para esta enfermedad se evaluó el potencial de distribución en México. Para ello, se tomó en cuenta factores que pueden causar una epidemia: 1) la idoneidad de las condiciones climáticas para la roya de la soya, 2) superficie sembrada con cultivos hospederos y 3) cantidad de inoculo. Para esto se cuantificaron los días favorables para la infección utilizando un modelo con datos meteorológicos diarios de temperatura, precipitación y humedad relativa de todo el país, para clasificar las zonas agrícolas de acuerdo a la probabilidad de ocurrencia de condiciones favorables para la infección de roya asiática en verano e invierno. Los resultados indicaron que en verano se tienen más días con condiciones favorables para la infección en los estados de Tamaulipas, Veracruz, Yucatán, Chiapas, Oaxaca, Guerrero, Michoacán, Jalisco, Nayarit, Sinaloa y Sonora. En temporada de invierno el número de días favorables disminuyeron de forma considerable. Basado en los resultados podemos concluir que la probabilidad de que la roya asiática hiberne en México es baja.
Key words: Phakopsora pachyrhizi, soya, roya soya, análisis de riesgo
INTRODUCTION
Soybean rust (SBR) is one of the most destructive diseases of soybeans in Asia and America (Christiano and Scherm 2007). Yield losses caused by SBR range from 10 to 90 % (Sharma and Gupta 2006) depending on the host susceptibility, inoculum quantity, and environmental conditions (Twizeyimana et ai 2011). The pathogen responsible for SBR is the fungus Phakopsora pachyrhizi Syd. &. P. Syd that was first reported in Japan in 1902 (Park et ai 2008). For decades, this disease was reported only in the eastern hemisphere, but in the last twenty years, the fungus was detected in new areas (Bonde et ai 2007). In 1994, the disease was located in Hawaii, and the first report of the disease in Africa was in Uganda by the end of 1996 (Levy 2005). In 2001, P. pachyrhizi was found infecting soybeans in Paraguay and Brazil and one year later was detected in Argentina (Rossi 2003). By 2004, the fungus was reported in Colombia, Ecuador and the United States (Pan et ai 2006), and in 2005, it was reported in Mexico, infecting soybeans and yam beans (Carcamo-Rodriguez and Aguilar-Rios 2006, Yanez-Morales et ai 2009). In Mexico, yield losses caused by SBR range from 25 to 80 % (Teran-Vargas et ai 2007) in both crops, which makes chemical control necessary.
Phakopsora pachyrhizi is an obligate parasite. Therefore, fungal survival depends on continued production of uredospores on a suitable host (Edwards and Bonde 2011). Phakopsora pachyrhizi naturally infects 95 species from 42 genera of legumes (Slaminko et ai 2008), but the limits of its host range are unknown. The reported hosts for this pathogen include soybeans (Glycine max) and yam beans (Pachyrhizus erosus). When these crops are severely infected, plants show early defoliation and a reduction in green leaf area, which directly affects yield (Hartman et ai 1991, Yang et ai 1992). Dry beans (Phaseolus vulgaris) are another important host and could play an important role in the pathogen spread because of its extensive area of cultivation in Mexico.
According to the epidemiological disease triangle, three factors determine the occurrence of a disease: the environment, a susceptible host, and the pathogen. These components interactively influence the complex and dynamic nature of the disease (Bonde et a/. 2007). Moreover, evidence shows that epidemic components, environmental conditions and meteorological factors have the greatest roles in affecting SBR epidemiology (Melching et ai 1989, Isard et ai 2006, Bonde et aI. 2007). Thus, meteorological factors affect the host and pathogen directly or indirectly, which results in complex interactions. For P. pachyrhizi, specific temperature and humidity conditions provide a stimulus to which the fungus responds at specific stages of its life cycle (Kochman 1979).
In the characterization of P. pachyrhizi, important advances have been achieved. The processes of spore germination, infection, latent period, lesion expansion and sporulation are influenced by meteorological variables (Marchetti et ai 1975, 1976, Kochman 1979, Melching et ai 1989). In general, moisture (rainfall and dew) and temperature affect important features of disease behavior such as successful initial establishment, infection efficiency, and the rate of development of the epidemic (Bonde et ai 2007). Pest risk analysis (PRA), based on science, is used to estimate the likelihood of entry, establishment and spread of a harmful organism in a defined area, and includes an impact evaluation (Pivonia et ai 2005, Pivonia and Yang 2006, Gutierrez and Ponti 2011). The use of modeling techniques in PRA is an emerging trend that assists the search for a more comprehensive and quantitative analysis. The modeling approaches to predict SBR epidemics vary from simple equations, such as linear regression, to complex algorithms such as neural networks and mechanistic models of population dynamics. According to Del Ponte et al. (2006a), SBR modeling is classified into two groups, simulation and empirical models. The simulation models are based on concepts derived from a pathosystem and reproduce biological processes such as the disease cycle, dispersion, and airborne inoculum deposition over long distances. These models are used to estimate the likelihood of entry of a pest into a region and the severity of damage that may be caused to hosts (Kim et al. 2005, Pan et al. 2006, Pivonia and Yang 2006). The empirical models are typically constructed through statistical relationships of explanatory variables that employ field data and are built using techniques such linear and nonlinear regression, neural networks and fuzzy logic (Reis et al. 2004, Kim et al. 2005, Del Ponte et al. 2006b). These models have commonly been used in disease forecasting systems that are based on variables such as temperature, rainfall, leaf wetness and relative humidity (Del Ponte et al. 2006a).
To assess the risk of soybean rust around the world, Pivonia and Yang (2004) estimated the likelihood of year-round survival of P. pachyrhizi and found that the main regions where SBR was not reported, but where it might be overwintering, were located in the western hemisphere and included northern South America, Central America, the Caribbean, Mexico, southern Texas, and Florida. The dry season should limit the survival in regions such as north and central Mexico and some parts of southeastern Africa (Pivonia and Yang 2004). In a more recent study, Pivonia et al. (2005) concluded that the potential overwintering areas were primarily located in the eastern coastal regions, including the Gulf of Mexico. This study developed a deductive mapping approach based on the analysis of the climatic conditions and the host densities to delimit the most suitable zones for the infection of P. pachyrhizi. These zones are at the highest risk of developing infections of soybean rust.
MATERIALS AND METHODS
To identify the municipalities where SBR was reported, the positive detections of SBR in Mexico were integrated. The host availability was incorporated using statistical data of planted areas with the susceptible hosts in summer and winter seasons. Subsequently, the climate analysis of potential distribution zones for soybean rust was performed using the SIMPEC software developed by Quijano et al. (2011), which integrated a daily climatic time series for Mexico and a platform to construct and calibrate crop and pest models. With the purpose to evaluate the suitability for P. pachyrhizi in zones where the susceptible hosts were planted, it was assumed that the inoculum was not a limiting factor. The favorable days for infection were determined through the analysis of daily meteorological data of temperature, rainfall, and relative humidity (RH). The final map was generated by overlay of host and climatic suitability maps.
SBR positive detection in Mexico
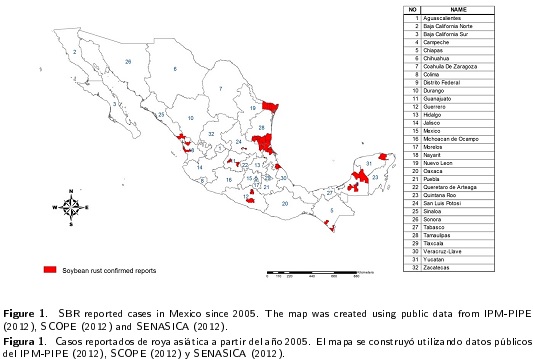
To identify the municipalities where SBR was reported, several web sources were consulted. These web sources were as follows: The Pest Information Platform for Extension and Education (IPM-PIPE), which is a web mapping site that provided the chronologic information from soybean rust observations, management recommendations, and scouting information (IPM - PIPE 2012); The Sistema Coordinado para la Vigilancia de Plagas Reglamentadas y su Epidemiología (SCOPE), which provided information on pest detection in Mexico, including SBR (SCOPE 2012); and the Servicio Nacional de Sanidad Inocuidad y Calidad Agroalimentaria (SENASICA), which constituted the plant heath authority in Mexico and was responsible for the official reports on the status of SBR in Mexico (SENASICA 2012). This information was used to build the map of all confirmed cases of SBR. The municipalities with confirmed reports of this disease were mapped in ARC-GIS ver. 10.1, using Mexico’s municipal divisions (Figure 1).
Host availability map
Host availability was incorporated using the statistics for planted areas in two crop seasons, summer and winter. For these seasons, host data for the municipality for soybeans (Glycine max), yam beans (Pachyrhizus erosus), common beans (Phaseolus vulgaris), and fava beans (Vicia faba) were combined with planted area data of 2010 that was reported by the Servicio de Información Agroalimentaria y Pesquera of Mexico (SIAP 2011). The host map was overlaid on the land use map to visualize only the agricultural areas of the municipalities.
Climatic time series
Weather data were obtained from the Servicio Meteorológico Nacional of Mexico (SMN-CONAGUA). The daily data of the meteorological variables rainfall, maximum temperature, and minimum temperature were used from 2 791 weather stations. The observed values of these variables represented a series from 1950 to 2010. Data quality control was performed through an exhaustive visual inspection of yearly graphics for each variable. After the elimination of invalid or illogical values, the data from 2 470 weather stations with a time series of at least 18 yr in the period from 1967 to 2008 were considered. Minimum and maximum temperature and daily rainfall data were used to run the model. The RH was calculated using potential evaporation as suggested by Ortiz (1993) as a function of evapotranspiration. The potential evaporation calculation required a daytime temperature approximation and the soil-plant reflection coefficient for solar radiation. The daytime temperature approximation was obtained using the weighted mean of the daily maximum and minimum air temperatures. The combined crop and soil albedo was calculated from the leaf area index, and the model calculated the input of bare soil albedo (Ritchie 1998).
Climatic conditions analysis
Uredospore germination occurred on plant surfaces only when free water was present. This process might be stimulated by different conditions. Under wet conditions, spore germination initiated in a minimum of three hours and increased with dew period (Marchetti et al. 1975, Melching et al. 1989). Spore germination was strongly affected by temperature if moisture was not limiting. Temperatures below 9 °C or above 28 °C inhibited uredospore germination, and the optimum temperature range was from 20 to 25 °C (Marchetti et al. 1975). The establishment of infection required a minimum of 6 h of moisture at a temperature of 24 °C. Additionally, an increment in the leaf wetness duration at 85 and 95 % RH increased the number of pustules per lesion and the lesion size. The lesion size on leaf blades increased after 12 h of leaf wetness at 21 °C and 85 % RH.
To calculate the number of days with favorable climatic conditions for uredospore infection, daily RH and temperature data were used. Two growing seasons were analyzed, the summer season (april to October) and the winter season (november to march).
The temperature effect on SBR infection was assumed to follow a classical response curve for biological systems with three cardinal temperatures: optimum, maximum, and minimum. According to the model, the risk index had a value of zero when the temperature was below 10 °C and above 28 °C and reached the maximum value when the temperature was near 22 °C. According to Eccel (2012), this occurred only when rain was more than 1 mm or RH was higher than 80 %. Optimum conditions and cardinal temperatures for the fungus based on literature values determined the infection index values. The infection probability in field was typically high during the night and early in the morning because the relative humidity was higher and the leaf wetness duration was longer in the night and morning than in the day. Therefore, the infection index for each day was determined using the minimum temperature. When the analysis was performed on a daily level, the model returned a value between zero and one. These values were then summarized over time. Accumulated days with favorable conditions in each season were divided by the total number of days in the period to generate the Index of Suitability for SBR (ISSBR). The curve of exceeding probability was calculated for every station, and the indexes that corresponded to 80 % probability were used to develop the maps using the software Arc map. Ordinary Kriging interpolation with a spherical semivariogram model was used to calculate the spatial distribution of the data. The climate risk maps represented the frequency of favorable conditions for P. pachyrhizi infection in the study area.
Favorable conditions and host maps
The overlay of host and climatic suitability maps generated the final map. The municipalities without areas planted with a host were excluded. The land use layer (INEGI) of the map was used to represent the host distribution in agricultural areas. These areas were used as a filter to visualize the ISSBR in the agricultural areas of the municipalities.
RESULTS
In Mexico, according to the statistics, host availability represented 7.9 % in the summer and 1.5 % in the winter of the total agricultural area in Mexico. However, according to the references consulted, this soybean disease was only detected in 40 municipalities in 10 states of Mexico (Figure 1). The study showed that, compared with the summer, the winter season presented a low frequency of favorable conditions for infection. By contrast, the summer season presented the most extensive planted area with susceptible hosts and the largest zone under infection risk along the coast of the Gulf of Mexico (Figure 2).
Seasonal analyses
Winter season. The weather conditions from november to march were analyzed. In general, the north and center of Mexico had a low frequency of favorable conditions. The agricultural areas located in the states of Baja California, Baja California Sur, Durango, Sonora, Sinaloa, Zacatecas, Aguascalientes, Jalisco, Guanajuato, the north of Michoacan, Queretaro, Hidalgo, Puebla, Tlaxcala, Mexico state, and the central zone of Oaxaca had frequencies of days with favorable conditions in the range from 0 to 0.3. This area represented the 60 % of the agricultural area. The frequencies of favorable days between 0.3 and 0.5 were reached in the states of Nayarit, southern Tamaulipas, San Luis Potosi, northern Veracruz, northern Guerrero, and central Chiapas. This area represented the 22 % of the agricultural land. The coastal areas of southern Veracruz, Tabasco, Yucatan, Quintana Roo, and the pacific coasts of Oaxaca, Guerrero and Michoacan presented the highest values, with 0.9 of the days with favorable conditions (Figure 2a).
Summer season. The weather conditions from may to October were analyzed. In general, coastal areas of Mexico had a high frequency of days with favorable conditions. The agricultural areas located in the states of Baja California, Chihuahua, Durango, Zacatecas, Aguascalientes, the north of Jalisco, Guanajuato, Queretaro, Hidalgo, Mexico, Tlaxcala, the north of Michoacan, Puebla and the west of Guerrero had frequencies of days with favorable conditions in the range of 0 to 0.3. These areas represented 50 % of the agricultural land. Favorable conditions were reached on 0.3 to 0.6 of the days, primarily in the states of Baja California Sur, Monterrey, central Guerrero, central Chiapas, Jalisco, and northern Guerrero. These areas represented 14 % of the agricultural land. The coastal areas of Tamaulipas, Veracruz, Yucatan, and the pacific coast of Chiapas, Oaxaca, Guerrero, Michoacan, Jalisco, Nayarit, Sinaloa and Sonora had the higher values, in the range from 0.6 to 1.0 of the days with favorable conditions (Figure 2b).
Monthly analysis. Figure 3 shows the frequency of days with favorable weather conditions for P. pachyrhizi infection from november to april. In november (Figure 3a), important areas with values from 0.6 to 1.0 were identified. These areas were the coastal zones of the states of Veracruz, southern Tamaulipas, Tabasco, Quintana Roo, Chiapas, Oaxaca, Guerrero, Michoacan, and Nayarit. Whereas in december, january, and february (Figures 3b, 3c, and 3d, respectively), favorable conditions decreased in the northern zones of Tamaulipas and Nayarit but increased again in march (Figure 3e) and april (Figure 3f), with values reaching nearly 1.0 along the Gulf of Mexico coast.
Figure 4 shows the frequency of days with favorable weather conditions for P. pachyrhizi infection from may to October (summer season). In may (Figure 4a), the highest risk remained in the same zones as in the previous month (april). Nearly all the agricultural zones near the seacoast around the country reached values of 1.0 for the infection risk in the months of june, july, august, and September (Figures 4b, 4c, 4d, and 4e, respectively). In October (Figure 4f), these values slightly decreased to 0.6 to 0.7 in most of these zones. By contrast, the high altitude central zones of the country presented conditions of low risk (values near zero) throughout the year.
DISCUSSION
Limiting the introduction of invasive species is of paramount importance. When a new species is discovered, it is important to accurately estimate its potential invasiveness, geographic distribution, and the possibility of eradication. These factors are important to preserve ecosystems (Parker et al. 1999, Gutierrez and Ponti 2011). The soybean rust caused by P. pachyrhizi is a clear example of an invasive disease that generated great controversy and expectations of harm, particularly after the soybean epidemics caused by P. pachyrhizi in the United States.
Since 2005, SBR was reported in restricted areas of municipalities of five states of Mexico: Tamaulipas, Veracruz, San Luis Potosí, Chiapas, and Campeche (Figure 1). From this date until present, Tamaulipas was the only state that reported the disease annually, and SBR was reported in Veracruz infecting crops of soybeans and yam beans since 2006. San Luis Potosi reported the presence of SBR from 2005 until present, except in the year 2009. The disease was confirmed in Chiapas in the years 2008, 2009 and 2011. The SBR was confirmed in Campeche only in the years 2006 and 2011 (SENASICA 2012, IPM-PIPE 2012, SCOPE 2012) and was reported in the states of Tamaulipas, San Luis Potosi, Veracruz, Campeche and Chiapas. The results of the analysis were consistent with these observations. As shown, states near the Gulf of Mexico had the highest favorable conditions of temperature and moisture for infection. Similarly, the south coastal states near the Pacific Ocean had high favorable conditions for infection.
Based on the sites analyzed, the period from November to March had a greater number of days with conditions for the disease as well as a greater area with susceptible hosts compared with the period from may to October. This meant that in winter the weather conditions and host densities were less favorable for overwintering of the fungus. These results were opposite to those proposed by Pivonia et al. (2005). Only the central Pacific areas had suitable conditions for SBR hibernation; however, the reduced area of hosts planted produced a shorter period with a favorable climate for infections. Two variables, temperature and long periods of moisture (rain, dew or relativity humidity) caused the increase in areas with favorable weather for infection. These conditions gave the fungus enough moisture for spore germination and increased the probability of infection. Additionally, the larger area of planted hosts increased the probability of spore interception in crops.
The different rates obtained generated information that was relevant to the management of SBR in Mexico. Although SBR was found in Mexico in soybeans and yam beans, the planted area of these crops was relatively low both in winter and summer seasons compared with common bean (SIAP 2011). The real danger for Mexican agriculture depends on the confirmation of the susceptibility of the common bean because in Mexico area planted about 1 506 033 ha (SIAP 2011). In addition to the low availability of susceptible hosts, the less favorable weather conditions in the winter season lead us to speculate that the risk that SBR might overwintering in Mexico is very small. However, efforts must be maintained in the disease response characterization of the Mexican common bean genotypes and in the general surveillance of the weather-host-pathogen interactions.
These studies help to establish the foundation for integrated risk modeling, which has been somewhat neglected in Mexico, despite being a fundamental part in the planning and the implementation of campaigns to detect disease or to control pest populations implemented by institutions whose focus is this problem. The spatial modeling of risk areas is important to understand the potential presence and distribution of any pest or disease. By using the temperature, humidity and the potential presence of hosts, digital maps give us the first approach to the modeling of the potential infection of P. pachyrhizi as is performed in countries like United States of America, Brazil and others (Parker et al. 1999, Pivonia et al. 2005, Gutierrez and Ponti 2011). The results contribute to the definition of areas of risk for the disease, indicate possible areas of infestation and identify areas to monitor for pest dispersion, and by their use in decision-making for planning, help to optimize the economic and human resources for management of the problem in the country by institutions focused on this as it is the National Health Service, Food Safety and Quality (SENASICA) in Mexico.
CONCLUSIONS
Specific and spatial modeling of risk areas is central to the understanding of the potential presence of any pest or disease. The analysis of favorable conditions for the infection of P. pachyrhizi in the different cycles of production allowed us to determine that the greatest risk for this disease occurs in the spring-summer season and to a lesser extent in the autumn-winter season. This is due to the combination of favorable temperatures and high relative humidity in the rainy period from may to november.
The results contribute to the definition of areas of risk for the disease, indicate possible areas of infestation and identify areas to monitor for pest dispersion, and by their use in decision-making for planning, help to optimize the economic and human resources for management of the problem in the country. This information can also be useful for the planning of strategies for phytosanitary campaigns with the aim of controlling the disease. Additionally, the results of this study provide a reference to determine the sites and planting dates for susceptible hosts, to avoid the co-occurrence of the crop cycle with favorable weather conditions for this disease.
ACKNOWLEDGEMENT
The authors acknowledge The Consejo Nacional de Ciencia y Tecnologia of Mexico for fellowship support of Ricardo Yanez Lopez.
LITERATURE CITED
Bonde MR, Berner DK, Nester SE, Frederick RD (2007) Effects of temperature on urediniospore germination, germ tube growth, and initiation of infection in soybean by Phakopsora isolates. Phytopathology 97: 997-1003.
Carcamo-Rodríguez A, Aguilar-Ríos J (2006) First report of Asian soybeans rust caused by Phakopsora pachyrhizi from México. Plant Disease 90: 1260-1260.
Christiano RCS, Scherm H (2007) Quantitative aspects of the spread of Asian soybean rust in the southeastern United States, 2005 to 2006. Phytopathology 97: 1428-1433.
Del Ponte EM, Godoy CV, Canteri MG, Reis EM, Yang XB (2006a) Models and applications for risk assessment and prediction of Asian soybean rust epidemics. Fitopatología Brasileira 31: 533-544.
Del Ponte EM, Godoy CV, Li X, Yang XB (2006b) Predicting severity of Asian soybean rust epidemics with empirical rainfall models. Phytopathology 96: 797-803.
Eccel E (2012) Estimating air humidity from temperature and precipitation measures for modeling applications. Meteorological Application 19: 118-128.
Edwards HH, Bonde MR (2011) Penetration and establishment of Phakopsora pachyrhizi in soybean leaves as observed by transmission electron microscopy. Phytopathology 101: 894-900.
Gutierrez AP, Ponti L (2011) Assessing the invasive potential of the Mediterranean fruit fly in California and Italy. Biological Invasions 13: 2661-2676.
Hartman GL, Wang TC, Tschanz AT (1991) Soybean rust development and the quantitative relationship between rust severity and soybean yield. Plant Disease 75: 596-600.
IPM-PIPE (2012) Integrated Pest Management - Pest information for Extension and Education. Available: http://sbr.ipmpipe.org/cgi-bin/sbr/public.cgi. Data consulted: november 25, 2013.
Isard SA, Dufault NS, Miles MR, Hartman GL, Russo JM, De Wolf ED, et al (2006) The effect of solar irradiance on the mortality of Phakopsora pachyrhizi urediniospores. Plant Disease 90: 941-945.
Kim KS, Wang TC, Yang XB (2005) Simulation of apparent infection rate to predict severity of soybean rust using a fuzzy logic system. Phytopathology 95: 1122-1131.
Koch man JK (1979) The effect of temperature on development of soybean rust (Phakopsora pachyrhizi). Australian Journal of Agricultural Research 30: 273-277.
Levy C (2005) Epidemiology and chemical control of soybean rust in Southern Africa. Plant Disease 89: 669-674.
Marchetti MA, Melching JS, Bromfield, K. R. (1976) The effects of temperature and dew period on germination and infection by uredospores of Phakopsora pachyrhizi. Phytopathology 66: 461-463.
Marchetti MA, Uecker FA, Bromfield KR (1975) Uredial development of Phakopsora pachyrhizi in soybean. Phytopathology 65: 822-823.
Melching JS, Dowler WM, Koogle DL, Royer MH (1989) Effects of duration, frequency, and temperature of leaf wetness periods on soybean rust. Plant Disease 73: 117-122.
Ortiz, SCA (1993) Elementos de Agrometeorología cuantitativa con aplicaciones en la República Mexicana. 2nd Edition. UACH. Departamento de Suelos. Chapingo, México 235p.
Pan Z, Yang XB, Pivonia S, Xue L, Pasken R (2006) Long-term prediction of soybean rust entry into the continental United States. Plant Disease 90: 840-846.
Park S, Chen ZY, Chanda AK, Schneider RW, Hollier CA (2008) Viability of Phakopsora pachyrhizi urediniospores under simulated southern Louisiana winter temperature conditions. Plant Disease 92: 14561462.
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1: 3-19.
Pivonia S, Yang XB (2004) Assessment of the potential year-round establishment of soybean rust throughout the world. Plant Disease 88: 523-529.
Pivonia S, Yang XB, Pan Z (2005) Assessment of epidemic potential of soybean rust in the United States. Plant Disease 89: 678-682.
PivoniaS, Yang XB (2006) Relating epidemic progress from a general disease model to seasonal appearance time of rusts in the United States: Implications for soybean rust. Phytopathology 96: 400-407.
Quijano CJA, Rocha RR, Aguirre GJA (2011) Sistema de información para el monitoreo del potencial ecológico de los cultivos, SIMPEC. Instituto Nacional de Investigaciones Forestales, Agricolas y Pecuarias. Publicación Técnica Núm. 2. Mexico 32p.
Reis EM, Sartori AF, Camara R K (2004) Modelo climático para a previsáo da ferrugem da soja. Summa Phytopathologica 30: 290-292.
Ritchie JT, 1998. Soil water balance and plant water stress. In: Tsuji GY, Hoogenboom G, and Thornton PK (eds). Understanding options of agricultural production. Kluwer Academic Publishers and International Consortium for Agricultural Systems Applications. The Netherlands, pp: 41-53.
Rossi RL (2003) First report of Phakopsora pachyrhizi, the causal organism of soybean rust in the Province of Misiones, Argentina. Plant Disease 87: 102.
SCOPE (2012) Sistema coordinado de operaciones para el manejo de plagas reglamentadas y su epidemiología. https://scopepublico.zedxinc.com/cgi-bin/index.cgi. Data consulted: november 13, 2013.
SENASICA (2012) Ficha Técnica Phakopsora pachyrizi Sydow &. Sydow Roya asiática de la soya. Available: http://www.senasica.gob.mx/includes/asp/download.asp?ldDocumento=19732&ldUrl=32204. Data consulted: november 2, 2013.
Sharma SK, Gupta GK (2006) Current status of soybean rust (Phakopsora pachyrhizi) - A Review. Agriculture Reviews 27: 91-102.
SIAP (2011) Servicio de Información Agroalimentaria y Pesquera Secretaría de Agricultura. Sistema de Producción agrícola. Avalilable:http://www.siap.gob.mx/index.php?option=com_wrapper&view=wrapper<emid=351. Data consulted: January 13, 2013.
Slaminko TL, Miles MR, Frederick RD, Bonde MR, Hartman GL (2008) New legume hosts of Phakopsora pachyrhizi based on greenhouse evaluations. Plant Disease 92: 767-771.
Terán-Vargas AP, Ascencio GL, Maldonado MN, Ávila JV (2007) La roya asiática de la soya en México. Folleto Técnico No. 22. Campo Experimental Sur de Tamaulipas, CIRNE-INIFAP. Mexico. 53 p.
Twizeyimana M, Ojiambo PS, Hartman GL, Bandyopadhyay R (2011) Dynamics of soybean rust epidemics in sequential plantings of soybean cultivars in Nigeria. Plant Disease 95: 43-50.
Yang XB, Dowler WM, Tschanz AT, Wang TC (1992) Comparing the effects of rust on plot yield, plant yield, yield components, and vegetative parts of soybean. Journal of Phytopathology 136: 45-56.
Yáñez-Morales MJ, Alaniz-Martínez I, Soto-Rocha JM, Malvick DK, Kurle JE, Floyd CM, et al. (2009) Soybean rust caused by Phakopsora pachyrhizi detected in the estate of Campeche on the Yucatan peninsula, Mexico. Plant Disease 93: 847.